Release Notes:
Measure Information Form
Version 2010B
**NQF-ENDORSED VOLUNTARY CONSENSUS STANDARDS FOR HOSPITAL CARE**Measure Information Form
Measure Set: Perinatal Care(PC)
Set Measure ID: PC-05
Performance Measure Name: Exclusive Breast Milk Feeding
Description: Exclusive breast milk feeding during the newborn's entire hospitalization
Rationale: Exclusive breast milk feeding for the first 6 months of neonatal life has long been the expressed goal of World Health Organization (WHO), Department of Health and Human Services (DHHS), American Academy of Pediatrics (AAP) and American College of Obstetricians and Gynecologists (ACOG). ACOG has recently reiterated its position (ACOG, 2007). A recent Cochrane review substantiates the benefits (Kramer et al., 2002). Much evidence has now focused on the prenatal and intrapartum period as critical for the success of exclusive (or any) BF (Centers for Disease Control and Prevention [CDC], 2007; Petrova et al., 2007; Shealy et al., 2005; Taveras et al., 2004). Exclusive breast milk feeding rate during birth hospital stay has been calculated by the California Department of Public Health for the last several years using newborn genetic disease testing data. Healthy People 2010 and the CDC have also been active in promoting this goal.
Type of Measure: Process
Improvement Noted As: Increase in the rate
Numerator Statement: Newborns that were fed breast milk only since birth
Included Populations: Not applicable
Excluded Populations: None
Data Elements:
Denominator Statement: Term newborns discharged from the hospital
Included Populations:
Live-born newborns with ICD-9-CM Principal Diagnosis Code or ICD-9-CM Other Diagnosis Codes for term gestation as defined in Appendix A, Table 11.20.1
Excluded Populations:
- Admitted to the Neonatal Intensive Care Unit (NICU) at this hospital during the hospitalization
- ICD-9-CM Principal Diagnosis Code or ICD-9-CM Other Diagnosis Codes for galactosemia as defined in Appendix A, Table 11.21
- ICD-9-CM Principal Procedure Code or ICD-9-CM Other Procedure Codes for parenteral infusion as defined in Appendix A, Table 11.22
- Experienced death
- Length of Stay >120 days
- Enrolled in clinical trials
- Documented Reason for Not Exclusively Feeding Breast Milk
Data Elements:
Continuous Variable Statement:
Included Populations:
Excluded Populations:
Data Elements:
Risk Adjustment: No.
Data Collection Approach: Retrospective data sources for required data elements include administrative data and medical records.
Data Accuracy: Variation may exist in the assignment of ICD-9-CM codes; therefore, coding practices may require evaluation to ensure consistency.
Measure Analysis Suggestions: In order to identify areas for improvement in breast milk feeding rates, hospitals may wish to review documentation for reasons. Education efforts can be targeted based on the specific reasons identified.
Sampling: Yes. For additional information see the Sampling Section.
Data Reported As: Aggregate rate generated from count data reported as a proportion.
Selected References:
- American Academy of Pediatrics. (2005). Section on Breastfeeding. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics.115:496 506.
- American College of Obstetricians and Gynecologists. (Feb. 2007). Committee on Obstetric Practice and Committee on Health Care for Underserved Women.Breastfeeding: Maternal and Infant Aspects. ACOG Committee Opinion 361.
- California Department of Public Health. (2006). Genetic Disease Branch. California In-Hospital Breastfeeding as Indicated on the Newborn Screening Test Form, Statewide, County and Hospital of Occurrence: Available at: http://www.cdph.ca.gov/data/statistics/Pages/BreastfeedingStatistics.aspx.
- Centers for Disease Control and Prevention. (Aug 3, 2007). Breastfeeding trends and updated national health objectives for exclusive breastfeeding--United States birth years 2000-2004. MMWR - Morbidity & Mortality Weekly Report. 56(30):760-3.
- Centers for Disease Control and Prevention. (2007). Division of Nutrition, Physical Activity and Obesity. Breastfeeding Report Card. Available at: http://www.cdc.gov/breastfeeding/data/report_card2.htm.
- Ip, S., Chung, M., Raman, G., et al. (2007). Breastfeeding and maternal and infant health outcomes in developed countries. Rockville, MD: US Department of Health and Human Services. Available at: http://www.ahrq.gov/downloads/pub/evidence/pdf/brfout/brfout.pdf
- Kramer, M.S. & Kakuma, R. (2002).Optimal duration of exclusive breastfeeding. [107 refs] Cochrane Database of Systematic Reviews. (1):CD003517.
- Petrova, A., Hegyi, T., & Mehta, R. (2007). Maternal race/ethnicity and one-month exclusive breastfeeding in association with the in-hospital feeding modality. Breastfeeding Medicine. 2(2):92-8.
- Shealy, K.R., Li, R., Benton-Davis, S., & Grummer-Strawn, L.M. (2005).The CDC guide to breastfeeding interventions. Atlanta, GA: US Department of Health and Human Services, CDC. Available at: http://www.cdc.gov/breastfeeding/pdf/breastfeeding_interventions.pdf.
- Taveras, E.M., Li, R., Grummer-Strawn, L., Richardson, M., Marshall, R., Rego, V.H., Miroshnik, I., & Lieu, T.A. (2004). Opinions and practices of clinicians associated with continuation of exclusive breastfeeding. Pediatrics. 113(4):e283-90.
- US Department of Health and Human Services. (2007). Healthy People 2010 Midcourse Review. Washington, DC: US Department of Health and Human Services. Available at: http://www.healthypeople.gov/data/midcourse.
- World Health Organization. (1991). Indicators for assessing breastfeeding practices. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/child-adolescent-health/new_publications/nutrition/who_cdd_ser_91.14.pdf.
Original Performance Measure Source / Developer:
California Maternal Quality Care Collaborative
Measure Algorithm:
 Attach file Attach file
|
Measure Information Form PC-05
Specifications Manual for Joint Commission National Quality Core Measures (2010B)
Discharges 10-01-10 (4Q10) through 03-31-11 (1Q11)
|
|
|

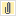 Attach file
Attach file