Measure Information Form
Version 2023A1
Comprehensive Stroke (CSTK)
Set Measures
| Set Measure ID | Measure Short Name |
|---|---|
| CSTK-01 | National Institutes of Health Stroke Scale (NIHSS Score Performed for Ischemic Stroke Patients) |
| CSTK-02 | Modified Rankin Score (mRS at 90 Days) |
| CSTK-03 | Severity Measurement Performed for SAH and ICH Patients (Overall Rate) |
| CSTK-04 | Procoagulant Reversal Agent Initiation for Intracerebral Hemorrhage (ICH ) |
| CSTK-05 | Hemorrhagic Transformation (Overall Rate) |
| CSTK-06 | Nimodipine Treatment Administered |
| CSTK-07 | Median Time to Revascularization |
| CSTK-08 | Thrombolysis in Cerebral Infarction (TICI Post-Treatment Reperfusion Grade) |
| CSTK-09 | Arrival Time to Skin Puncture (Overall Rate) |
| CSTK-10 | Modified Rankin Score (mRS at 90 Days: Favorable Outcome) |
| CSTK-11 | Rate of Rapid Effective Reperfusion From Hospital Arrival |
| CSTK-12 | Rate of Rapid Effective Reperfusion From Skin Puncture |
General Data Elements
| Element Name | Collected For |
|---|---|
| Admission Date | All Records, |
| Birthdate | All Records, |
| Discharge Date | All Records, Not collected for HBIPS-2 and HBIPS-3 |
| Hispanic Ethnicity | All Records, |
| ICD-10-CM Other Diagnosis Codes | All Records, Optional for HBIPS-2, HBIPS-3 |
| ICD-10-CM Principal Diagnosis Code | All Records, Optional for HBIPS-2, HBIPS-3 |
| ICD-10-PCS Other Procedure Codes | All Records, Optional for All HBIPS Records |
| ICD-10-PCS Other Procedure Dates | All Records, Optional for All HBIPS Records |
| ICD-10-PCS Principal Procedure Code | All Records, Optional for All HBIPS Records |
| ICD-10-PCS Principal Procedure Date | All Records, Optional for All HBIPS Records |
| Measure Category Assignment | All Records, Calculation, Used in calculation of the Joint Commission's aggregate data. |
| Payment Source | All Records, Optional for HBIPS-2 and HBIPS-3 |
| Race | All Records, |
| Sex | All Records, |
Algorithm Output Data Elements
| Element Name | Collected For |
|---|---|
| Measure Category Assignment | All Records, Calculation |
| Measurement Value | Calculation |
Measure Set Specific Data Elements
Related Materials
Comprehensive Stroke (CSTK) Initial Patient Population
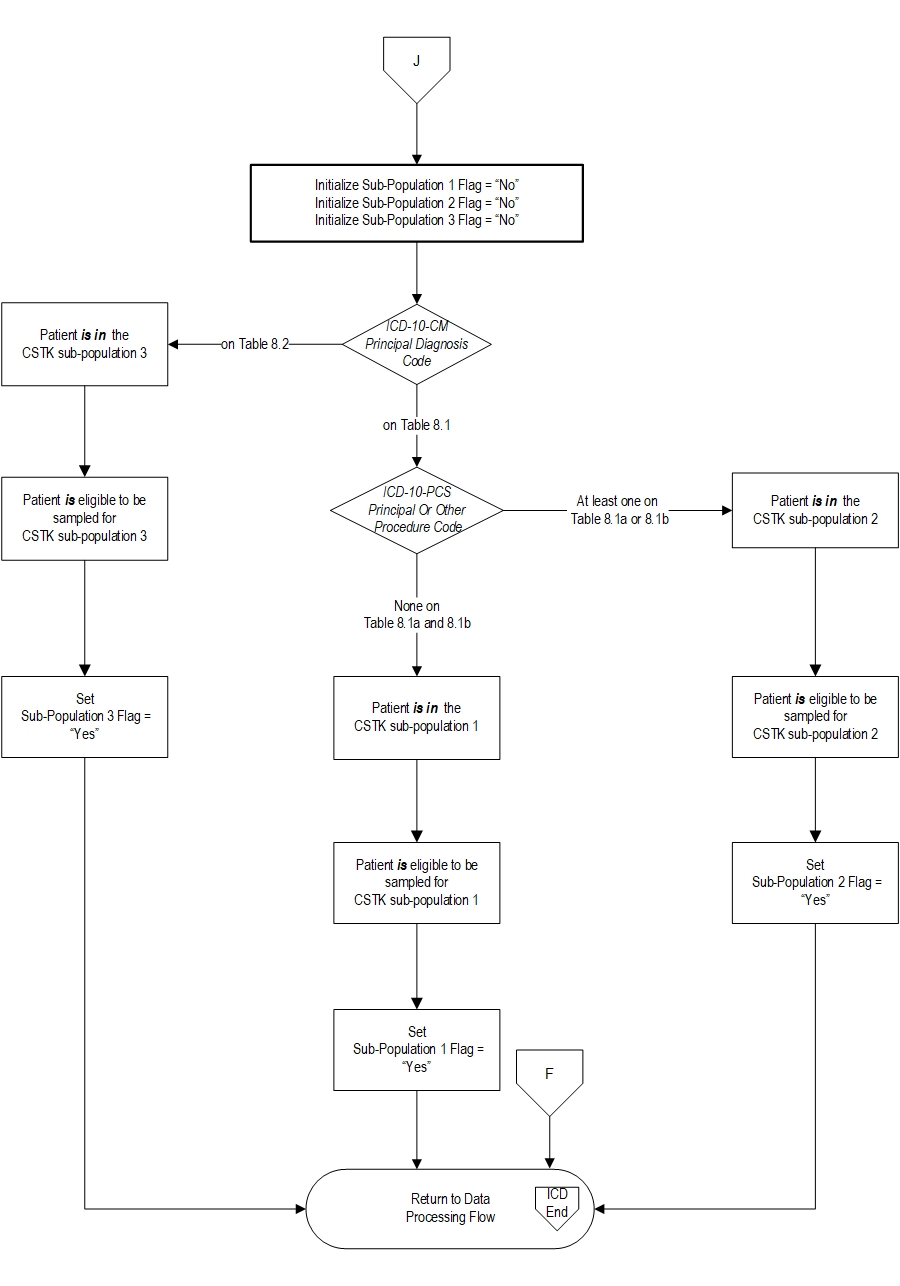
The CSTK Initial Patient Population is unique in that it is comprised of three distinct subpopulations: ischemic stroke patients who do not undergo a reperfusion therapy (i.e., procedure), ischemic stroke patients who undergo a reperfusion therapy (IV t-PA, IA t-PA, or mechanical endovascular reperfusion (MER) therapy), and hemorrhagic stroke patients.
Ischemic StrokeThe population of the CSTK 1-Ischemic Stroke measures (CSTK-01) are identified using 4 data elements:
- Admission Date
- Birthdate
- Discharge Date
- ICD-10-CM-Principal Diagnosis Code
Patients admitted to the hospital for inpatient acute care are included in the CSTK 1-Ischemic Stroke Without Procedure subpopulation sampling group if they have: ICD-10-CM Principal Diagnosis Code as defined in Appendix A, Table 8.1, a Patient Age (Admission Date – Birthdate) ≥ 18 years and a Length of Stay (Discharge Date - Admission Date) ≤ 120 days.
Note: Hospitals are NOT required to sample their data. If sampling offers minimal benefit (e.g., a hospital has 45 cases for the quarter and must select a sample of 42 cases), the hospital may choose to use all cases. Ischemic Stroke With IV t-PA, IA t-PA, or MERThe population of the CSTK 2-Ischemic Stroke With IV t-PA, IA t-PA, or MER measures (CSTK-01, CSTK-02, CSTK-05, CSTK-07, CSTK-08, CSTK-09, CSTK-10, CSTK-11, CSTK-12) are identified using 5 data elements:
- Admission Date
- Birthdate
- Discharge Date
- ICD-10-CM Principal Diagnosis Code
- ICD-10-PCS Principal or Other Procedure Codes
Patients admitted to the hospital for inpatient acute care are included in the CSTK-2 Ischemic Stroke With IV t-PA, IA t-PA, or MER subpopulation sampling group if they have: ICD-10-CM Principal Diagnosis Code as defined in Appendix A, Table 8.1 AND ICD-10-PCS Principal or Other Procedure Codes as defined in Appendix A, Table 8.1a OR Table 8.1b, a Patient Age (Admission Date – Birthdate) ≥ 18 years and a Length of Stay (Discharge Date - Admission Date) ≤ 120 days.
Note: Hospitals are NOT required to sample their data. If sampling offers minimal benefit (e.g., a hospital has 45 cases for the quarter and must select a sample of 42 cases), the hospital may choose to use all cases. Hemorrhagic StrokeThe population of the CSTK 3-Hemorrhagic Stroke measures (CSTK-03, CSTK-04, CSTK-06) are identified using 4 data elements:
- Admission Date
- Birthdate
- Discharge Date
- ICD-10-CM-Principal Diagnosis Code
Patients admitted to the hospital for inpatient acute care are included in the CSTK 3-Hemorrhagic Stroke subpopulation sampling group if they have: ICD-10-CM Principal Diagnosis Code as defined in Appendix A, Table 8.2, a Patient Age (Admission Date – Birthdate) ≥ 18 years and a Length of Stay (Discharge Date - Admission Date) ≤ 120 days.
Note: Hospitals are NOT required to sample their data. If sampling offers minimal benefit (e.g., a hospital has 80 cases for the quarter and must select a sample of 75 cases), the hospital may choose to use all cases.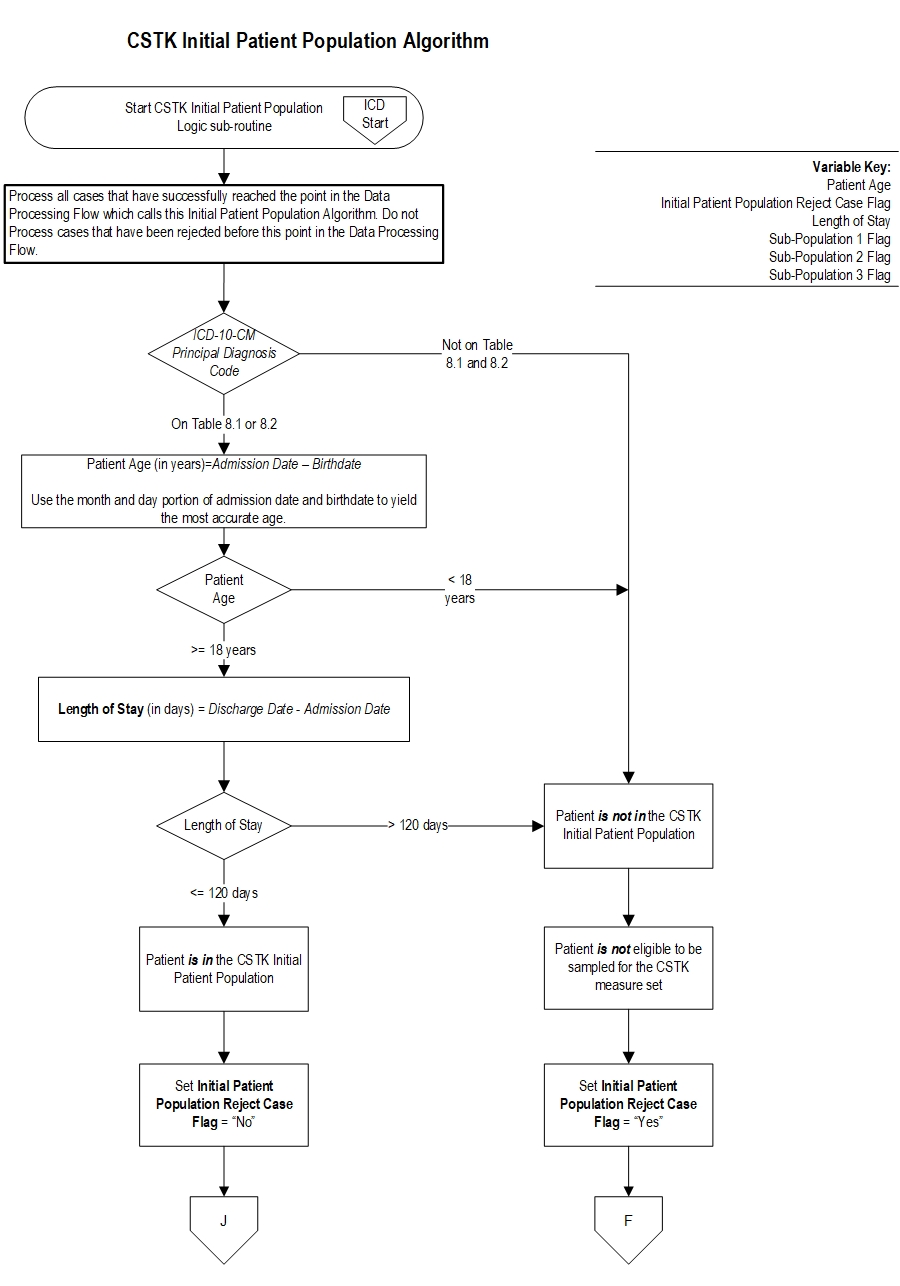
CSTK Sample Size Requirements
Hospitals that choose to sample have the option of sampling quarterly or sampling monthly. A hospital may choose to use a larger sample size than is required. Hospitals whose Initial Patient Population size is less than the minimum number of cases per quarter for the measure set cannot sample.
Regardless of the option used, hospital samples must be monitored to ensure that sampling procedures consistently produce statistically valid and useful data. Due to exclusions, hospitals selecting sample cases MUST submit AT LEAST the minimum required sample size.
The following sample size tables for each option automatically build in the number of cases needed to obtain the required sample sizes. For information concerning how to perform sampling, refer to the Population and Sampling Specifications section in this manual.
Quarterly Sampling
Hospitals performing quarterly sampling for CSTK must ensure that its Initial Patient Population and sample size meet the following conditions for each sampling group:Based on CSTK Subpopulation 1 for Ischemic Stroke Without Procedure
(CSTK-01 Measure)
Hospital’s Measure (Table 1)
| Average Quarterly Initial Patient Population Size "N" |
Minimum Required Sample Size "n" |
|---|---|
| > 420 | 84 |
| 211-419 | 20% of Patient Population size |
| 43-210 | 42 |
| ≤ 42 | No sampling; 100% Patient Population required |
Based on CSTK Subpopulation 2 for Ischemic Stroke With IV t-PA, IA t-PA,or MER
(CSTK-01, CSTK-02, CSTK-05, CSTK-07, CSTK-08, CSTK-09, CSTK-10, CSTK-11, CSTK-12)
Hospital’s Measure (Table 2)
| Average Quarterly Initial Patient Population Size "N" |
Minimum Required Sample Size "n" |
|---|---|
| > 420 | 84 |
| 211-419 | 20% of Patient Population size |
| 43-210 | 42 |
| ≤ 42 | No sampling; 100% Patient Population required |
Based on CSTK Subpopulation 3 for Hemorrhagic Stroke
(CSTK-03, CSTK-04, CSTK-06)
Hospital’s Measure (Table 3)
| Average Quarterly Initial Patient Population Size "N" |
Minimum Required Sample Size "n" |
|---|---|
| > 750 | 150 |
| 376-750 | 20% of Patient Population size |
| 76-375 | 75 |
| ≤ 75 | No sampling; 100% Patient Population required |
Monthly Sampling
Hospitals performing monthly sampling for CSTK must ensure that its Initial Patient Population and sample size meet the following conditions for each sampling group:Based on CSTK Subpopulation 1 for Ischemic Stroke Without Procedure
(CSTK-01 Measure)
Hospital’s Measure (Table 4)
| Average Monthly Initial Patient Population Size "N" |
Minimum Required Sample Size "n" |
|---|---|
| > 140 | 28 |
| 71-140 | 20% of Patient Population size |
| 15-70 | 14 |
| ≤ 14 | No sampling; 100% Patient Population required |
Based on CSTK Subpopulation 2 for Ischemic Stroke With IV t-PA, IA t-PA, or MER
(CSTK-01, CSTK-02, CSTK-05, CSTK-07, CSTK-08, CSTK-09, CSTK-10, CSTK-11, CSTK-12)
Hospital’s Measure (Table 5)
| Average Monthly Initial Patient Population Size "N" |
Minimum Required Sample Size "n" |
|---|---|
| > 140 | 28 |
| 71-140 | 20% of Patient Population size |
| 15-70 | 14 |
| ≤ 14 | No sampling; 100% Patient Population required |
Based on CSTK Subpopulation 3 for Hemorrhagic Stroke
(CSTK-03, CSTK-04, CSTK-06)
Hospital’s Measure (Table 6)
| Average Monthly Initial Patient Population Size "N" |
Minimum Required Sample Size "n" |
|---|---|
| > 250 | 50 |
| 126-250 | 20% of Patient Population size |
| 26-125 | 25 |
| ≤ 25 | No sampling; 100% Patient Population required |
Sample Size Examples
NOTE: Two subpopulations make-up the population for the CSTK-01 measure; CSTK Subpopulation 1 for Ischemic Stroke Without Procedure and CSTK Subpopulation 2 for Ischemic Stroke With IV t-PA, IA t-PA, or MER. Both sampling groups must be sampled to meet the minimum sampling requirement for CSTK-01.Examples:
- A hospital’s ischemic stroke patient population size is 200 patients during the second quarter. Fifty (50) ischemic stroke patients had a procedure for thrombolysis or mechanical clot removal. The required quarterly sample size for the CSTK-01 measure is a minimum of 84 cases (42 cases from Table 1 plus 42 cases from Table 2 equals 84).
- A hospital’s ischemic stroke patient population size is 200 patients during March. Twenty (20) ischemic stroke patients had a procedure for thrombolysis or mechanical clot removal. The required sample size for the CSTK-01 measure is a minimum of 42 cases for the month (28 cases from Table 4 plus 14 cases from Table 5 equals 42).
Quarterly sampling
CSTK Subpopulation 1 for Ischemic Stroke Without Procedure
- A hospital’s ischemic stroke patient population size is 495 cases during the second quarter. Using the quarterly sampling table for the ischemic stroke subpopulation, the sample size required is 84 cases for the quarter.
- A hospital’s ischemic stroke patient population size is 392 cases during the second quarter. Using the quarterly sampling table for the ischemic stroke subpopulation, the sample size required is 20% of this subpopulation or 78 cases for the quarter (20% of 392 equals 78.4 rounded to the next highest whole number equals 78).
- A hospital’s ischemic stroke patient population size is 200 cases during the second quarter. Using the quarterly sampling table for the ischemic stroke subpopulation, the sample size required is 42 cases for the quarter.
- A hospital’s ischemic stroke patient population size is 37 cases during the second quarter. Using the quarterly sampling table for the ischemic stroke subpopulation, the sample size is less than the minimum required quarterly sample size, so 100% of the subpopulation or all 37 cases are sampled.
- Four-hundred and twenty-eight (428) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during the second quarter. Using the quarterly sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size required is 84 cases for the quarter.
- Two-hundred and twenty-three (223) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during the second quarter. Using the quarterly sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size required is 20% of this subpopulation or 45 cases for the quarter (20% of 223 equals 44.6 rounded to the next highest whole number equals 45).
- Fifty (50) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during the second quarter. Using the quarterly sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size required is 42 cases for the quarter.
- Nineteen (19) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during the second quarter. Using the quarterly sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size is less than the minimum required quarterly sample size, so 100% of the subpopulation or all 19 cases are sampled.
- A hospital’s hemorrhagic stroke patient population size is 795 cases during the second quarter. Using the quarterly sampling table for the hemorrhagic stroke subpopulation, the sample size required is 150 cases for the quarter.
- A hospital’s hemorrhagic stroke patient population size is 392 cases during the second quarter. Using the quarterly sampling table for the hemorrhagic stroke subpopulation, the sample size required is 20% of this subpopulation or 78 cases for the quarter (20% of 392 equals 78.4 rounded to the next highest whole number equals 78).
- A hospital’s hemorrhagic stroke patient population size is 200 cases during the second quarter. Using the quarterly sampling table for the hemorrhagic stroke subpopulation, the sample size required is 75 cases for the quarter.
- A hospital’s hemorrhagic stroke patient population size is 67 cases during the second quarter. Using the quarterly sampling table for the hemorrhagic stroke subpopulation, the sample size is less than the minimum required quarterly sample size, so 100% of the subpopulation or all 67 cases are sampled.
Monthly sampling
CSTK Subpopulation 1 for Ischemic Stroke Without Procedure
- A hospital’s ischemic stroke patient population size is 295 cases during March. Using the monthly sampling table for the ischemic stroke subpopulation, the sample size required is 28 cases for the month.
- A hospital’s ischemic stroke patient population size is 129 cases during March. Using the monthly sampling table for the ischemic stroke subpopulation, the sample size required is 20% of this subpopulation or 26 cases for the month (20% of 129 equals 25.8 rounded to the next highest whole number equals 26).
- A hospital’s ischemic stroke patient population size is 70 cases during March. Using the monthly sampling table for the ischemic stroke subpopulation, the sample size required is 14 cases for the month.
- A hospital’s ischemic stroke patient population size is 7 cases during March. Using the monthly sampling table for the ischemic stroke subpopulation, the sample size is less than the minimum required monthly sample size, so 100% of the subpopulation or all 7 cases are sampled.
- One-hundred and forty-eight (148) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during March. Using the monthly sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size required is 28 cases for the month.
- One-hundred and twenty-three (123) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during March. Using the monthly sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size required is 20% of this subpopulation or 25 cases for the month (20% of 123 equals 24.6 rounded to the next highest whole number equals 25).
- Sixty (60) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during March. Using the monthy sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size required is 14 cases for the month.
- Eleven (11) ischemic stroke cases had IV or IA thrombolysis or a mechanical clot removal procedure during March. Using the monthly sampling table for the ischemic stroke with IV t-PA, IA t-PA or MER subpopulation, the sample size is less than the minimum required monthly sample size, so 100% of the subpopulation or all 11 cases are sampled.
- A hospital’s hemorrhagic stroke patient population size is 295 cases during March. Using the monthly sampling table for the hemorrhagic stroke subpopulation, the sample size required is 50 cases for the month.
- A hospital’s hemorrhagic stroke patient population size is 129 cases during March. Using the monthly sampling table for the hemorrhagic stroke subpopulation, the sample size required is 20% of this subpopulation or 26 cases for the month (20% of 129 equals 25.8 rounded to the next highest whole number equals 26).
- A hospital’s hemorrhagic stroke patient population size is 60 cases during March. Using the monthly sampling table for the hemorrhagic stroke subpopulation, the sample size required is 25 cases for the month.
- A hospital’s hemorrhagic stroke patient population size is 17 cases during March. Using the monthly sampling table for the hemorrhagic stroke subpopulation, the sample size is less than the minimum required monthly sample size, so 100% of the subpopulation or all 17 cases are sampled.
CPT® only copyright 2022 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.