Release Notes:
Measure Information Form
Version 2016A1
Advanced Certification Heart Failure Outpatient (ACHFOP)
Set Measures
| Set Measure ID |
Measure Short Name |
| ACHFOP-01 |
Hospital Outpatient Beta-Blocker Therapy (i.e., Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Succinate Prescribed for LVSD) |
| ACHFOP-02 |
Hospital Outpatient ACEI or ARB Prescribed for LVSD |
| ACHFOP-03 |
Hospital Outpatient Aldosterone Receptor Antagonists Prescribed for LVSD |
| ACHFOP-04 |
Hosptial Outpatient New York Heart Association (NYHA Classification Assessment) |
| ACHFOP-05 |
Hospital Outpatient Activity Recommendations |
| ACHFOP-06 |
Hospital Outpatient Discussion of Advance Directives/Advance Care Planning |
| ACHFOP-07 |
Hospital Outpatient Advance Directive Executed |
General Data Elements
Measure Set Specific Data Elements
| Element Name |
Collected For |
| ACEI Prescribed for LVSD in the Outpatient Setting |
ACHFOP-02, |
| ARB Prescribed for LVSD in the Outpatient Setting |
ACHFOP-02, |
| Activity Recommendation Duration of Activity |
ACHFOP-05, |
| Activity Recommendation Intensity of Activity |
ACHFOP-05, |
| Activity Recommendation Type of Activity |
ACHFOP-05, |
| Advance Directive Executed |
ACHFOP-07, |
| Aldosterone Receptor Antagonist Prescribed for LVSD in the Outpatient Setting |
ACHFOP-03, |
| Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Prescribed for LVSD in the Outpatient Setting |
ACHFOP-01, |
| Clinical Trial |
ACHFOP-01, ACHFOP-02, ACHFOP-03, ACHFOP-04, ACHFOP-05, |
| Discharge Code |
ACHFOP, |
| Discussion of Advance Directives/Advance Care Planning |
ACHFOP-06, |
| E/M Code |
ACHFOP, |
| LVSD < 40% |
ACHFOP-01, ACHFOP-02, ACHFOP-03, |
| New York Heart Association (NYHA) Classification |
ACHFOP-04, |
| Outpatient Encounter Date |
ACHFOP, |
| Reason for No ACEI and No ARB Prescribed for LVSD in Outpatient Setting |
ACHFOP-02, |
| Reason for No Activity Recommendations in the Outpatient Setting |
ACHFOP-05, |
| Reason for No Aldosterone Receptor Antagonist Prescribed for LVSD in the Outpatient Setting |
ACHFOP-03, |
| Reason for No Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Prescribed for LVSD in the Outpatient Setting |
ACHFOP-01, |
Related Materials
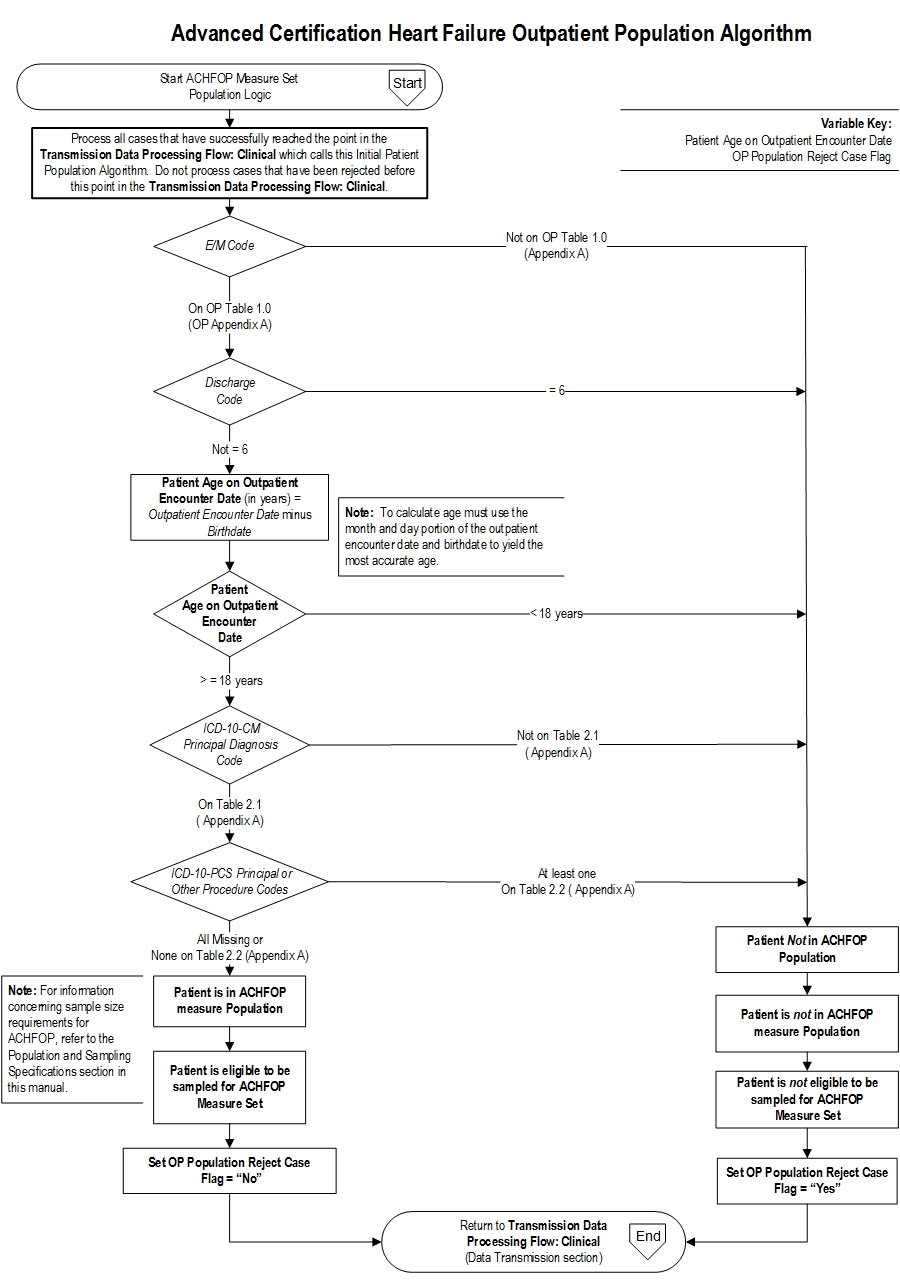
Initial Patient Population
HF Sample Size Requirements
Hospitals that choose to sample have the option of sampling quarterly or sampling monthly. A hospital may choose to use a larger sample size than is required. Hospitals whose Initial Patient Population size is less than the minimum number of cases per quarter for the measure set cannot sample. Hospitals that have five or fewer HF discharges (both Medicare and non-Medicare combined) in a quarter are not required to submit HF patient level data to the QIO Clinical Warehouse
1 and Joint Commissions Data Warehouse.
Regardless of the option used, hospital samples must be monitored to ensure that sampling procedures consistently produce statistically valid and useful data. Due to exclusions, hospitals selecting sample cases MUST submit AT LEAST the minimum required sample size.
The following sample size tables for each option automatically build in the number of cases needed to obtain the required sample sizes. For information concerning how to perform sampling, refer to the Population and Sampling Specifications section in this manual.
Quarterly Sampling
Hospitals performing quarterly sampling for HF must ensure that its Initial Patient Population and sample size meet the following conditions:
Quarterly Sample Size
Based on Initial Patient Population Size for the HF Measure Set
Hospital's Measure
Monthly Sampling
Hospitals performing monthly sampling for HF must ensure that its Initial Patient Population and sample size meet the following conditions:
Monthly Sample Size
Based on Initial Patient Population Size for the HF Measure Set
Hospital's Measure
Sample Size Examples
- Quarterly sampling:
- The HF Initial Patient Population size for a hospital has been 500 patients per quarter during the past year. The required quarterly sample size would be 100 (twenty percent of 500) heart failure patients per quarter -- as this number is smaller than the maximum condition (i.e., 304 cases) and larger than the minimum condition (i.e., 76 cases).
- A hospitals HF Initial Patient Population size is 1,482 patients during the third quarter. The required sample size is 20% of the patient population or 297 cases for the quarter (twenty percent of 1,482 equals 296.4 rounded to the next highest whole number equals 297).
- A hospitals HF Initial Patient Population size is 5 patients during the first quarter. Submission of patient level data is not required. If the hospital chooses to submit patient level data:
- CMS: the quarterly sample size would be 1 5 cases for the quarter.1
- The Joint Commission: the required quarterly sample size would be 100% of the patient population or 5 cases for the quarter.
- Monthly sampling:
- A hospitals HF Initial Patient Population size is 25 patients during March. Since this is less than the minimum condition (i.e., 26 cases), no sampling is allowed or 100% of the patient population of 25 cases is required.
- A hospitals HF Initial Patient Population size is 503 patients during July. The required sample size is 20% of the patient population or 101 cases for the month (twenty percent of 503 equals 100.6 rounded to the next highest whole number equals 101).
1 The Heart Failure (HF) core measure sampling methodology is applicable to both the HF core measures as well as the ACHF measures. The HF sampling methodology is shared by CMS and The Joint Commission; however, data collection for the ACHF measures is required by Joint Commission Disease-Specific Care Certification only.
Measure Information Form ACHFOP
Specifications Manual for Joint Commission National Quality Measures (v2016A1)
Discharges 10-01-16 through 12-31-16 (4Q16)
