Release Notes:
Measure Information Form
Version 2012B
**NQF-ENDORSED VOLUNTARY CONSENSUS STANDARDS FOR HOSPITAL CARE**Measure Information Form
Measure Set: Hospital Based Inpatient Psychiatric Services (HBIPS)
Set Measure ID: HBIPS-2
| Set Measure ID |
Performance Measure Name |
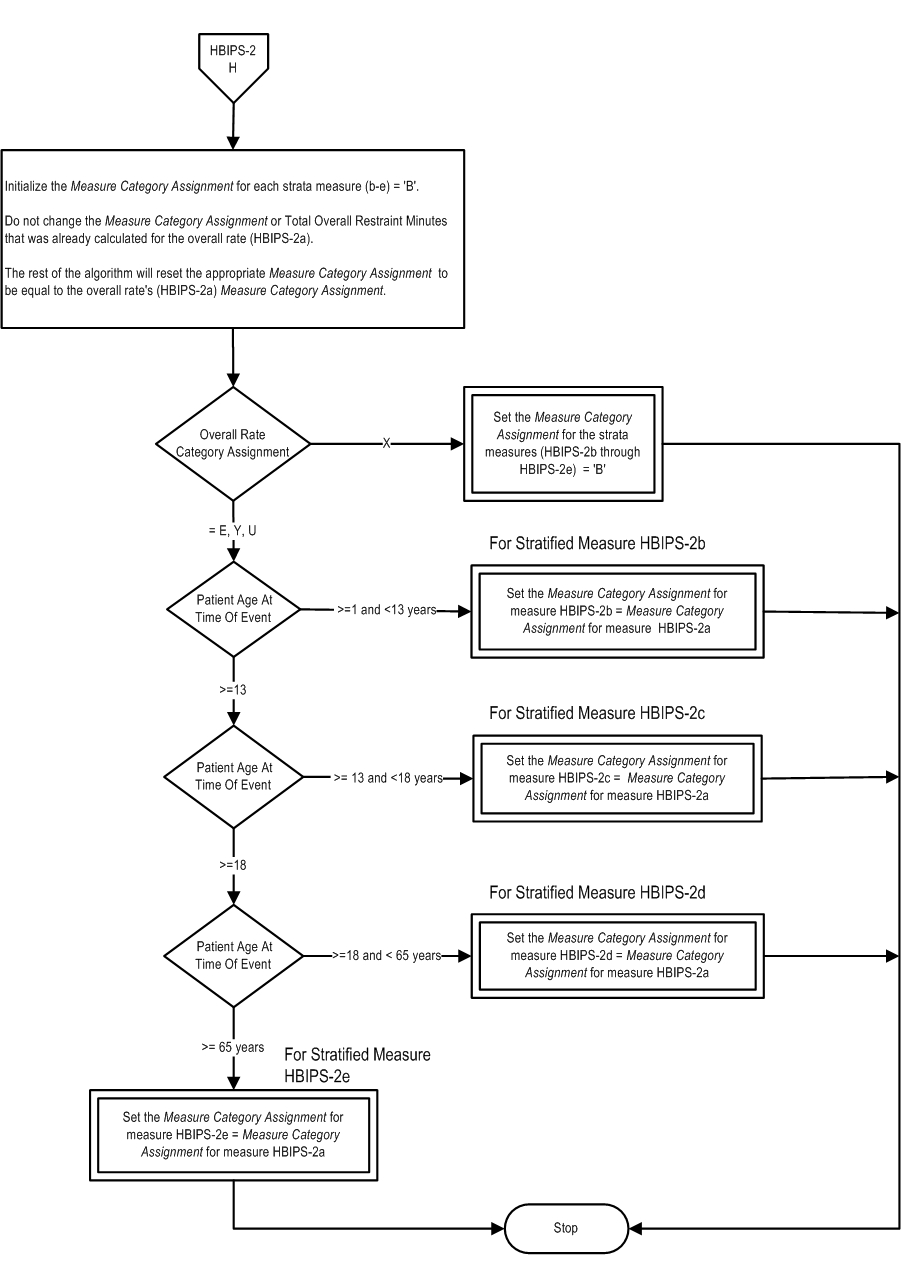
| HBIPS-2a |
Physical Restraint- Overall Rate |
| HBIPS-2b |
Physical Restraint- Children (1 through 12 years) |
| HBIPS-2c |
Physical Restraint- Adolescent (13 through 17 years) |
| HBIPS-2d |
Physical Restraint- Adult (18 through 64 years) |
| HBIPS-2e |
Physical Restraint- Older Adult (≥ 65 years) |
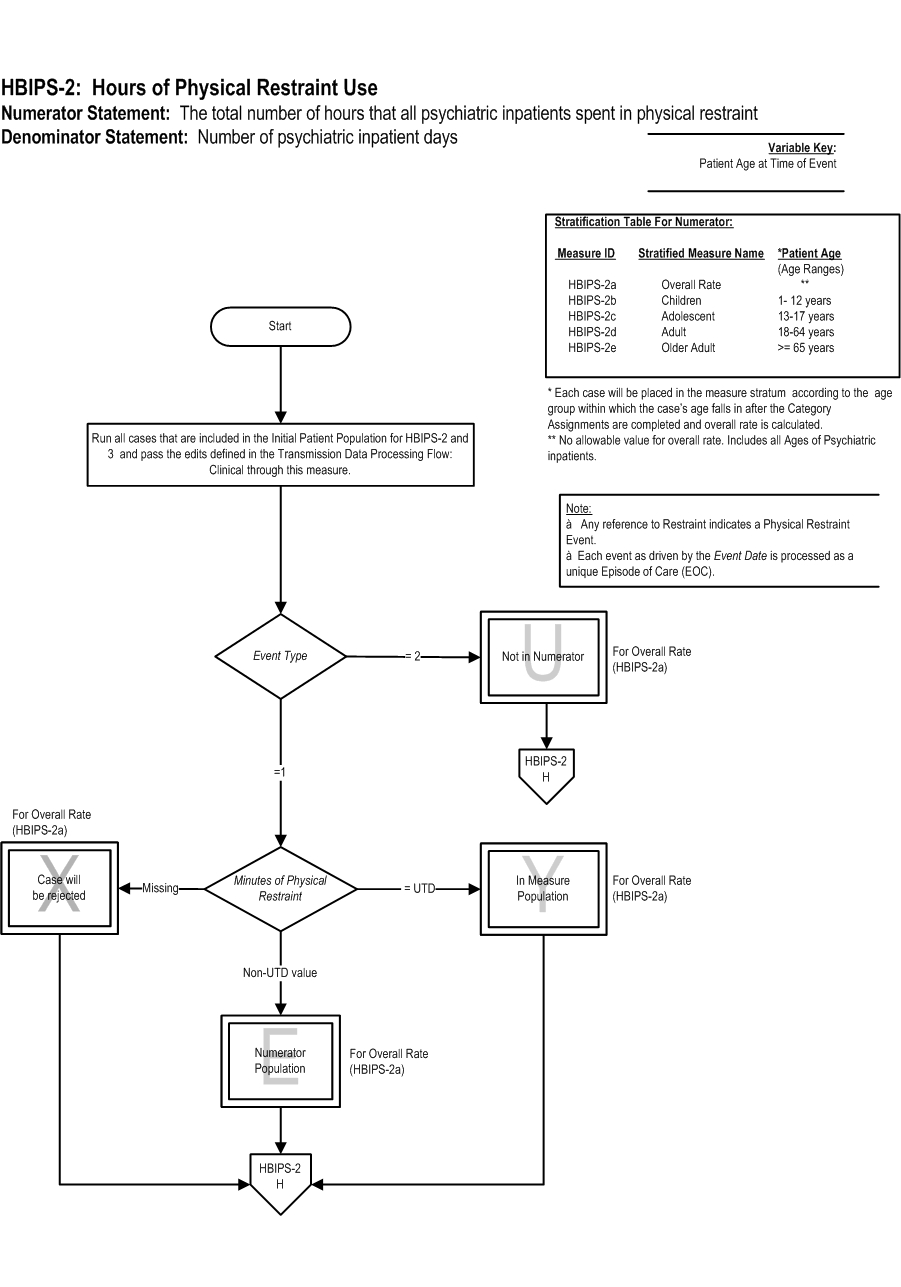
Performance Measure Name: Hours of physical restraint use
Description: The total number of hours that all patients admitted to a hospital-based inpatient psychiatric setting were maintained in physical restraint.
Rationale: Mental health providers that value and respect an individual’s autonomy, independence and safety seek to avoid the use of dangerous or restrictive interventions at all times (Donat, 2003). The use of seclusion and restraint is limited to situations deemed to meet the threshold of imminent danger and when restraint and seclusion are used; such use is rigorously monitored and analyzed to prevent future use. Providers also seek to prevent violence or aggression from occurring in their treatment environments by focusing their attention on prevention activities that have a growing evidence base (Donat, 2003).
Type of Measure: Process
Improvement Noted As: Decrease in the rate
Numerator Statement: The total number of hours that all psychiatric inpatients were maintained in physical restraint
Numerator Basis: The numerator evaluates the number of hours of physical restraint; however, the algorithm calculates the number of minutes to ensure a more accurate calculation of the measure. Convert the minutes to hours when analyzing and reporting this measure.
Included Populations:
- Patients for whom at least one physical restraint event is reported during the month
Excluded Populations: None
Data Elements:
Denominator Statement: Number of psychiatric inpatient days
Denominator Basis: per 1,000 hours
Included Populations:
- All psychiatric inpatient days
Excluded Populations:
Data Elements:
Continuous Variable Statement:
Included Populations:
Excluded Populations:
Data Elements:
- [[Manual.TableOfContents][]]
- [[Manual.SamplingHCSS][]]
- [[Manual.ReleaseRecord0014PublishRecord][]]
- [[Manual.ReleaseRecord0017PublishRecord][]]
- [[Manual.ReleaseNotes][]]
- [[Manual.DataCluster004Form][]]
- [[Manual.TypeOfMeasure05][]]
- [[Manual.UseStatus02][]]
- [[Manual.DataCluster009][]]
- [[Manual.TransmissionChapter][]]
- [[Manual.QuestionCategoryID17][]]
- [[Manual.SCR00000][]]
- [[Manual.ReleaseRecord0001][]]
- [[Manual.QuestionCategoryID03][]]
- [[Manual.QuestionCategoryID47][]]
- [[Manual.QuestionCategoryID38][]]
- [[Manual.RenderSpecificationChangeRequest][]]
- [[Manual.MissingDataChapter][]]
- [[Manual.SpecificationChangeRequestTemplate][]]
- [[Manual.AppendixDTJC][]]
- [[Manual.AppendixBTJC][]]
- [[Manual.ImpNoteAsValueID05][]]
- [[Manual.InternetExplorerIssues][]]
- [[Manual.ReleaseRecord0025PublishRecord][]]
- [[Manual.QuestionCategoryID62][]]
- [[Manual.TopicType][]]
- [[Manual.QuestionCategoryID03a][]]
- [[Manual.AdoptionStatus01][]]
- [[Manual.ReleaseRecord0019PublishRecord][]]
- [[Manual.QuestionCategoryID49][]]
- [[Manual.QuestionCategoryID21][]]
- [[Manual.GlossaryStroke][]]
- [[Manual.DataCluster011Form][]]
- [[Manual.ClassifiedTopic][]]
- [[Manual.HelpDocuments][]]
- [[Manual.DraValueID01][]]
- [[Manual.ReleaseRecord0005PublishRecord][]]
- [[Manual.WebAtom][]]
- [[Manual.QuestionCategoryID24][]]
- [[Manual.WebAliases][]]
- [[Manual.DataCluster013][]]
- [[Manual.UsingTheManualPBM][]]
- [[Manual.TableOfContentsPBM][]]
- [[Manual.ReleaseRecord0023PublishRecord][]]
- [[Manual.AppendixA][]]
- [[Manual.AppendixCTJC][]]
- [[Manual.AppendixH][]]
- [[Manual.CoverPagePBM][]]
- [[Manual.TransmissionAlphaDataDictionaryTJC][]]
- [[Manual.QuestionCategoryID15][]]
- [[Manual.QuestionCategoryID56][]]
- [[Manual.QuestionCategoryID58][]]
- [[Manual.DataUtilizationStroke][]]
- [[Manual.Introduction][]]
- [[Manual.UsingTheManual][]]
- [[Manual.DevStatus00][]]
- [[Manual.DataCluster013Form][]]
- [[Manual.QuestionCategoryID43][]]
- [[Manual.QuestionCategoryID13][]]
- [[Manual.DataCluster006][]]
- [[Manual.HealthcareFrameworkHCSS][]]
- [[Manual.CreateNewTopic][]]
- [[Manual.WebLinks][]]
- [[Manual.ImpNoteAsValueID03][]]
- [[Manual.QuestionCategoryID36][]]
- [[Manual.QuestionCategoryID26][]]
- [[Manual.DevStatus01][]]
- [[Manual.CommentID10002][]]
- [[Manual.QuestionCategoryID05][]]
- [[Manual.WebSearchAdvanced][]]
- [[Manual.FormAttributeValueTemplate][]]
- [[Manual.QuestionCategoryID60][]]
- [[Manual.SCRDetailsID100004][]]
- [[Manual.QuestionCategoryID53][]]
- [[Manual.Releases][]]
- [[Manual.DataUtilizationHCSS][]]
- [[Manual.ReleaseRecord0024PublishRecord][]]
- [[Manual.DataElement][]]
- [[Manual.DataCollectionApproach][]]
- [[Manual.BCCPopulationAndSampling][]]
- [[Manual.DataCluster006Form][]]
- [[Manual.QuestionCategoryID28][]]
- [[Manual.AppendixDPBM][]]
- [[Manual.QuestionCategoryID25][]]
- [[Manual.DataReportingToolStroke][]]
- [[Manual.AppendixCPBM][]]
- [[Manual.AppendixAAMICodeTables][]]
- [[Manual.QuestionCategoryID34][]]
- [[Manual.AppendixATableForm][]]
- [[Manual.PopulationOptionsID01][]]
- [[Manual.QuestionCategoryID52][]]
- [[Manual.PreOpAntibioticProphylaxis][]]
- [[Manual.MeasureInformationForm][]]
- [[Manual.DataCluster003][]]
- [[Manual.QuestionCategoryID01][]]
- [[Manual.ReleaseRecord0016PublishRecord][]]
- [[Manual.TableofContentsHCSS][]]
- [[Manual.TransmissionPopulationDataProcessingFlowTJC][]]
- [[Manual.DataCluster004][]]
- [[Manual.DataCluster][]]
- [[Manual.WebChanges][]]
- [[Manual.AppendixAPBM][]]
- [[Manual.DataReportedAs][]]
- [[Manual.StrokeGuidelines][]]
- [[Manual.TopicForm][]]
- [[Manual.IntroductionTJC][]]
- [[Manual.QuestionCategoryID41][]]
- [[Manual.QuestionCategoryID45][]]
- [[Manual.QuestionCategoryID57][]]
- [[Manual.WebCreateNewTopic][]]
- [[Manual.DataCluster002Form][]]
- [[Manual.VTEProphylaxisOptionsforSurgery][]]
- [[Manual.AlphaDataElementList][]]
- [[Manual.AppendixE][]]
- [[Manual.QuestionCategoryID11][]]
- [[Manual.TransmissionDataProcessingFlow][]]
- [[Manual.WebPreferences][]]
- [[Manual.QuestionCategoryID08][]]
- [[Manual.SpecificationChangeRequestForm][]]
- [[Manual.AppendixETJC][]]
- [[Manual.SCRDetailsID100002][]]
- [[Manual.QuestionCategoryID18][]]
- [[Manual.DataCollectionToolsHCSS][]]
- [[Manual.AppendixBPBM][]]
- [[Manual.TypeOfMeasure03][]]
- [[Manual.TransmissionAlphaDataDictionary][]]
- [[Manual.PublishPluginHistory][]]
- [[Manual.QuestionCategoryID32][]]
- [[Manual.AlgorithmOne][]]
- [[Manual.UseStatus][]]
- [[Manual.DataCollectionApproachID02][]]
- [[Manual.TableOfContentsTJC][]]
- [[Manual.TopicTree][]]
- [[Manual.DraValueID03][]]
- [[Manual.AppendixC][]]
- [[Manual.AppendixATJCAddendum][]]
- [[Manual.WebNotify][]]
- [[Manual.MeasureInformationFormTemplate][]]
- [[Manual.MeasureSetForm][]]
- [[Manual.SupplementalMaterialTemplate][]]
- [[Manual.ReleaseRecord0015PublishRecord][]]
- [[Manual.TransmissionDataProcessingFlowTJC][]]
- [[Manual.MeasureSet][]]
- [[Manual.AppendixStroke][]]
- [[Manual.ImpNoteAsValueID06][]]
- [[Manual.QuestionCategoryID39][]]
- [[Manual.ImpNoteAsValueID04][]]
- [[Manual.PopulationOptions][]]
- [[Manual.QuestionCategoryID48][]]
- [[Manual.DataCluster007][]]
- [[Manual.QuestionCategoryID04][]]
- [[Manual.AcknowledgementTJC][]]
- [[Manual.ReferencesHCSS][]]
- [[Manual.TransmissionChapterTJC][]]
- [[Manual.QuestionCategoryID61][]]
- [[Manual.QuestionCategoryID30][]]
- [[Manual.SCR00001][]]
- [[Manual.AdoptionStatus02][]]
- [[Manual.PublishedManuals][]]
- [[Manual.SCRDetailsID100003][]]
- [[Manual.DataCluster012][]]
- [[Manual.QuestionCategoryID29][]]
- [[Manual.SCR00002][]]
- [[Manual.QuestionCategoryID54][]]
- [[Manual.ReleaseRecord0020PublishRecord][]]
- [[Manual.WebAliasSearches][]]
- [[Manual.HelpGuideCoreMeasureSolutionExchange][]]
- [[Manual.TypeOfMeasure02][]]
- [[Manual.TypeOfMeasure][]]
- [[Manual.DataCluster010Form][]]
- [[Manual.PublishContribHistory][]]
- [[Manual.DataCluster008][]]
- [[Manual.DataCluster012Form][]]
- [[Manual.QuestionCategoryID16][]]
- [[Manual.DataCollectionApproachID03][]]
- [[Manual.IntroductionStroke][]]
- [[Manual.QuestionCategoryID44][]]
- [[Manual.SCRDetailsID100001][]]
- [[Manual.SamplingChapterTJC][]]
- [[Manual.IntroductionHCSS][]]
- [[Manual.SpecificationChangeRequestDetailsForm][]]
- [[Manual.AppendixHCSS][]]
- [[Manual.AppendixFTJC][]]
- [[Manual.ImpNoteAsValueID01][]]
- [[Manual.AppendixATJC][]]
- [[Manual.QuestionCategoryID33][]]
- [[Manual.UseStatus01][]]
- [[Manual.QuestionCategoryID37][]]
- [[Manual.ImpNoteAsValueID02][]]
- [[Manual.WebSearch][]]
- [[Manual.ImprovementNotedAs][]]
- [[Manual.CoverPageTJC][]]
- [[Manual.SpecificationChangeRequest][]]
- [[Manual.QuestionCategoryID23][]]
- [[Manual.WebStatistics][]]
- [[Manual.GlossaryHCSS][]]
- [[Manual.QualityMeasureVerificationProcess][]]
- [[Manual.QuestionCategoryID06][]]
- [[Manual.CommentID10003][]]
- [[Manual.CommentID10004][]]
- [[Manual.FormAttributeTemplate][]]
- [[Manual.QuestionCategoryID12][]]
- [[Manual.RestrictedAccessMessage][]]
- [[Manual.DataElementForm][]]
- [[Manual.QuestionCategoryID55][]]
- [[Manual.TypeOfMeasure04][]]
- [[Manual.FormAttributeForm][]]
- [[Manual.DataCluster005Form][]]
- [[Manual.DataCluster003Form][]]
- [[Manual.QuestionCategoryID14][]]
- [[Manual.DataCluster010][]]
- [[Manual.WebHome][]]
- [[Manual.WebIndex][]]
- [[Manual.DataDictionaryIntroduction][]]
- [[Manual.QuestionCategory][]]
- [[Manual.DataCluster014][]]
- [[Manual.DataCluster005][]]
- [[Manual.StaffNavigationMenu][]]
- [[Manual.ReleaseRecord0021PublishRecord][]]
- [[Manual.WebTopicList][]]
- [[Manual.DataCluster002][]]
- [[Manual.DataCollectionHCSS][]]
- [[Manual.ReleaseRecord0018PublishRecord][]]
- [[Manual.QuestionCategoryID35][]]
- [[Manual.SupplementalMaterial][]]
- [[Manual.QuestionCategoryID40][]]
- [[Manual.AdoptionStatus][]]
- [[Manual.DataCluster011][]]
- [[Manual.DataElementTemplate][]]
- [[Manual.QuestionCategoryID31][]]
- [[Manual.QuestionCategoryID00][]]
- [[Manual.QuestionCategoryID10][]]
- [[Manual.IntroductionPBM][]]
- [[Manual.MeasureSetPrintPackage][]]
- [[Manual.PublishContribHistoryTest][]]
- [[Manual.DataCluster009Form][]]
- [[Manual.AppendixD][]]
- [[Manual.AcknowledgementPBM][]]
- [[Manual.Acknowledgement][]]
- [[Manual.ReleaseRecord0026PublishRecord][]]
- [[Manual.QuestionCategoryID63][]]
- [[Manual.ReleaseRecord0008PublishRecord][]]
- [[Manual.QuestionCategoryID09][]]
- [[Manual.MeasureSetTemplate][]]
- [[Manual.CommentID10001][]]
- [[Manual.ReleaseRecord0022PublishRecord][]]
- [[Manual.WebSideBar][]]
- [[Manual.WebLeftBar][]]
- [[Manual.DataCluster008Form][]]
- [[Manual.ImpNoteAsValueID07][]]
- [[Manual.QuestionCategoryID20][]]
- [[Manual.QuestionCategoryID51][]]
- [[Manual.DataCollectionStroke][]]
- [[Manual.TableofContentsStroke][]]
- [[Manual.SamplingChapter][]]
- [[Manual.FormAttribute][]]
- [[Manual.QuestionCategoryID50][]]
- [[Manual.QuestionCategoryID07][]]
- [[Manual.RenderCreateNewSCRDetails][]]
- [[Manual.DataCluster014Form][]]
- [[Manual.PopulationOptionsID00][]]
- [[Manual.MissingAndInvalidDataTJC][]]
- [[Manual.StaffAssignment][]]
- [[Manual.RelatedTopicsList][]]
- [[Manual.DraValueID02][]]
- [[Manual.ProphylacticAntibioticRegimenSelectionForSurgery][]]
- [[Manual.QuestionCategoryID27][]]
- [[Manual.SCR00003][]]
- [[Manual.ReleaseRecord0027PublishRecord][]]
- [[Manual.DataDictionaryIntroductionPBM][]]
- [[Manual.ChangesToManual][]]
- [[Manual.QuestionCategoryID42][]]
- [[Manual.UseStatus00][]]
- [[Manual.SpecificationChangeRequestDetails][]]
- [[Manual.AppendixG][]]
- [[Manual.SpecificationChangeRequestDetailsTemplate][]]
- [[Manual.AppendixATableMap][]]
- [[Manual.TopicClassification][]]
- [[Manual.TestPublishTopic][]]
- [[Manual.QuestionCategoryID19][]]
- [[Manual.AlgorithmTwo][]]
- [[Manual.QuestionCategoryID46][]]
- [[Manual.SamplingStroke][]]
- [[Manual.QuestionCategoryID59][]]
- [[Manual.QuestionCategoryID22][]]
- [[Manual.TypeOfMeasure01][]]
- [[Manual.DataCollectionToolsStroke][]]
- [[Manual.DataCollectionApproachID01][]]
- [[Manual.DevelopmentStatus][]]
- [[Manual.ListOfDataElements][]]
- [[Manual.WebRss][]]
- [[Manual.UsingTheManualTJC][]]
- [[Manual.TopicClassForm][]]
- [[Manual.DataDictionaryIntroductionTJC][]]
- [[Manual.ReferencesStroke][]]
- [[Manual.QuestionCategoryID02][]]
- ABG Done
- ACEI Prescribed at Discharge
- ACEI Prescribed for LVSD in the Outpatient Setting
- ACEI or ARB for LVSD
- ACEI or ARB for LVSD
- ARB Prescribed at Discharge
- ARB Prescribed for LVSD in the Outpatient Setting
- Active Clinical Staff
- Active Labor
- Activity Recommendations Document
- Acute Myocardial Infarction
- Admission Date
- Admission Date-Stroke
- Admission From Home
- Admission Screening for Violence Risk, Substance Use, Psychological Trauma History and Patient Strengths completed
- Admission Screening- Adolescent (13 through 17 years)
- Admission Screening- Adult (18 through 64 years)
- Admission Screening- Children (1 through 12 years)
- Admission Screening- Older Adult (≥65 years)
- Admission Screening- Overall Rate
- Admission Time
- Admission Type
- Admission to NICU
- Admit Decision Time to ED Departure Time for Admitted Patients
- Admitted for Elective Carotid Endarterectomy
- Adult Smoking Cessation Advice/Counseling
- Adult Smoking Cessation Advice/Counseling
- Adult Smoking Cessation Advice/Counseling
- Adult Smoking Counseling
- Adult Smoking Counseling-Stroke
- Adult Smoking History
- Adult Smoking History-Stroke
- Advance Directives
- Advanced Certification Heart Failure
- Age
- Age 18 or Greater
- Alcohol & Drug Use: Assessing Status After Discharge
- Alcohol & Other Drug Use Disorder Treatment Provided or Offered at Discharge
- Alcohol & Other Drug Use Disorder Treatment Provided or Offered at Discharge
- Alcohol & Other Drug Use Disorder Treatment at Discharge
- Alcohol Use Brief Intervention
- Alcohol Use Brief Intervention Provided or Offered
- Alcohol Use Brief Intervention Provided or Offered
- Alcohol Use Screening
- Alcohol Use Status
- Alcohol or Drug Disorder
- Alcohol or Drug Use Status Post-Discharge (Not used in measure calculation)
- Aldosterone Receptor Antagonist Prescribed for LVSD in the Outpatient Setting
- Anesthesia Start Date
- Another Suspected Source of Infection
- Antenatal Steroid Administered
- Antenatal Steroids
- Antibiotic Administration Date
- Antibiotic Administration Route
- Antibiotic Administration Time
- Antibiotic Allergy
- Antibiotic Name
- Antibiotic Received
- Antibiotic Selection
- Antibiotic Timing
- Antibiotic Timing (Median)
- Antithrombotic Therapy Administered by End of Hospital Day Two
- Antithrombotic Therapy By End of Hospital Day Two
- Antithrombotic Therapy Prescribed at Discharge
- Appropriate Justification for Multiple Antipsychotic Medications
- Arrival Date
- Arrival Date
- Arrival Date-Stroke
- Arrival Time
- Arrival Time-Stroke
- Aspirin Prescribed at Discharge
- Aspirin Prescribed at Discharge
- Aspirin Received Within 24 Hours Before or After Hospital Arrival
- Aspirin at Arrival
- Aspirin at Arrival
- Assessed for Rehabilitation
- Assessed for Rehabilitation Services
- Assessment - Bone Health Risk
- Assessment - Fatigue
- Assessment - Lymphedema
- Assessment - Menopausal Symptoms
- Assessment - Neuropathy
- Assessment - Pain
- Assessment - Psychosocial Distress
- Assessment - Sleep
- Assessment for Chemotherapy Induced Nausea and Vomiting
- Assessment for Chemotherapy Induced Nausea and Vomiting
- Assessment for Distress
- Assessment for Fatigue
- Assessment for Sleep-Wake Disturbance
- Atrial Fibrillation
- Background Check
- Beta-Blocker Current Medication
- Beta-Blocker During Pregnancy
- Beta-Blocker Perioperative
- Beta-Blocker Prescribed at Discharge
- Beta-Blocker Prescribed at Discharge
- Beta-Blocker Received Within 24 Hours After Hospital Arrival
- Beta-Blocker Therapy (i.e., Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Succinate) for LVSD Prescribed at Discharge
- Beta-Blocker at Arrival
- Birth Weight
- Birthdate
- Birthdate
- Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Prescribed for LVSD in the Outpatient Setting
- Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Prescribed for LVSD at Discharge
- Blood Administration Documentation
- Blood Administration Location
- Blood Bank Records
- Blood Culture Collected
- Blood Cultures Performed Within 24 Hours Prior to or 24 Hours After Hospital Arrival for Patients Who Were Transferred or Admitted to the ICU Within 24 Hours of Hospital Arrival
- Blood Cultures Performed in the Emergency Department Prior to Initial Antibiotic Received in Hospital
- Blood ID Number
- Blood Type Testing Ordered
- Bone Mineral Density Test Performed in the 12 Months Prior to the Fracture
- Breast Cancer Care
- Breast Cancer Stage
- Breast Cancer Survivorship
- Breast Imaging
- Brief Intervention
- CMS Certification Number
- CMS Risk-Adjusted 30-Day Mortality
- CPT® Code
- CSF Prescribed
- Cardiac Arrest
- Cardiac Arrest Date
- Cardiac Arrest Event Number
- Cardiac Arrest Time
- Cardiac Resynchronization Therapy
- Cardiac Surgery Patients With Controlled 6 A.M. Postoperative Blood Glucose
- Care Transition Record Given to Patient/Caregiver
- Care Transition Record Present
- Care Transition Record Transmitted
- Care Transition Record-Condition or Functional Status at Discharge
- Care Transition Record-Designated Patient Caregiver or Social Support
- Care Transition Record-Discharge Medications
- Care Transition Record-Follow-Up Treatment and Services Needed
- Care Transition Record-Location, Date, and Time of Post-Discharge Appointment
- Care Transition Record-Next Level of Care Provider Contact Information
- Care Transition Record-Procedures Performed During Hospitalization
- Care Transition Record-Reason for Hospitalization
- Care Transition Record-Treatments/Services Provided
- Case Identifier
- Central Line-Associated Bloodstream Infection Rate
- Cesarean Section
- Cesarean Section - 15 through 19 years
- Cesarean Section - 20 through 24 years
- Cesarean Section - 25 through 29 years
- Cesarean Section - 30 through 34 years
- Cesarean Section - 35 through 39 years
- Cesarean Section - 40 through 44 years
- Cesarean Section - 45 through 64 years
- Cesarean Section - 8 through 14 years
- Cesarean Section - Overall Rate
- Chemotherapy Cycle Number
- Chest X-Ray
- Children’s Asthma Care
- Cholesterol Reducing Therapy Prescribed at Discharge
- Cholesterol Reducing Therapy Prior To Hospitalization
- Clinical Indication for Plasma
- Clinical Indication for Platelets
- Clinical Indication for Prophylactic Platelets
- Clinical Indication for RBCs
- Clinical Staff
- Clinical Trial
- Clinical Trial - Osteo
- Code Status Order
- Code Status Order Date
- Code Status Order Time
- Cognitive Impairment
- Colony Stimulation Factors (CSF) Prescribed
- Colorectal Surgery Patients with Immediate Postoperative Normothermia
- Comatose
- Comfort Measures Only
- Comfort Measures Only
- Comfort Measures Only-Stroke
- Competency
- Completeness of Personnel File
- Compromised
- Congenital Anomalies
- Consent Addressed Transfusion Benefits
- Consent Addressed Transfusion Risks
- Continuing Assessment
- Continuing Assessment - Distress
- Continuing Assessment - Fatigue
- Continuing Assessment - Overall Rate
- Continuing Assessment - Sleep-Wake Disturbance
- Continuing Care Plan-Discharge Medications
- Continuing Care Plan-Next Level of Care
- Continuing Care Plan-Principal Discharge Diagnosis
- Continuing Care Plan-Reason for Hospitalization
- Contraindication to Systemic Corticosteroids
- Contraindication to Aspirin at Discharge
- Contraindication to Aspirin on Arrival
- Contraindication to Beta-Blocker - Perioperative
- Contraindication to Beta-Blocker at Discharge
- Contraindication to Beta-Blocker on Arrival
- Contraindication to Both ACEI and ARB at Discharge
- Contraindication to Relievers
- Contraindication to VTE Prophylaxis
- Coordination of Care
- DVT Prophylaxis Initiated by End of Hospital Day 2
- DXA Scan Ordered or Performed Prior to Discharge
- Data Source
- Date
- Date IV Thrombolytic Therapy Administered At This Hospital
- Date Last Known Well
- Date Therapeutic Hypothermia Ended
- Date Therapeutic Hypothermia Initiated
- Date Therapeutic Hypothermia Ordered
- Date of Infection
- Death Among Surgical Inpatients with Treatable Serious Complications
- Diagnostic Uncertainty
- Discharge Date
- Discharge Date-SCA
- Discharge Disposition
- Discharge Instructions
- Discharge Instructions Address Activity
- Discharge Instructions Address Diet
- Discharge Instructions Address Follow-up
- Discharge Instructions Address Medications
- Discharge Instructions Address Symptoms Worsening
- Discharge Instructions Address Weight Monitoring
- Discharge Instructions – Emergency Department
- Discharge Status
- Discharge Status
- Discharge Status-Stroke
- Discharge Time
- Discharged Home From The ED
- Discharged on Antithrombotic Therapy
- Discharged on Cholesterol Reducing Medication
- Discussion of Advance Directives
- Disease Specific Instrument
- Distress
- Distress Improvement
- Do Not Return - Clinical
- Do Not Return - Clinical
- Do Not Return - Professional
- Do Not Return - Professional
- Do Not Return Occurrence Identifier
- Dysphagia Screen
- Dysphagia Screening
- E/M Code
- ED Patient
- Education Addresses Activation of Emergency Medical System
- Education Addresses Follow-up After Discharge
- Education Addresses Medication Prescribed
- Education Addresses Risk Factors for Stroke
- Education Addresses Warning Signs and Symptoms of Stroke
- Education on Community Resources
- Education on Diet
- Education on Exercise
- Education on Late Effects
- Education on Lymphedema
- Education on Neutropenia Precautions
- Education on Recurrence
- Elective Delivery
- Elective Surgery
- Emergency Department
- Emergency Order Date
- Emergency Order Time
- Emergency Transfusion
- Emergency Transfusion Date
- Emergency Transfusion Time
- Emetogenic Agents
- Endotracheal Intubation Confirmation Date
- Endotracheal Intubation Confirmation Time
- Endotracheal Intubation Date
- Endotracheal Intubation Time
- Endotracheal Tube Insertion
- Evaluation of LVS Function
- Event Date
- Event Type
- Exclusive Breast Milk Feeding
- Exclusive Breast Milk Feeding
- Exercise Program
- FDA-approved Pharmacotherapy for Treatment of Osteoporosis
- Falls with Injury
- Fatigue Improvement
- Fatigue Level Begin
- Fatigue Level End
- Fibrinolytic Administration
- Fibrinolytic Administration Date
- Fibrinolytic Administration Time
- Fibrinolytic Therapy Received Within 30 Minutes
- Fibrinolytic Therapy Received Within 30 Minutes of Hospital Arrival
- Final Treatment Date
- First Chemotherapy Date
- First Defibrillation Shock Date
- First Defibrillation Shock Time
- First In-Hospital LDL-Cholesterol Qualitative Description
- First In-Hospital LDL-Cholesterol Value
- First Name
- First PCI Date
- First PCI Time
- Follow Up Care
- Follow Up Care - Breast Imaging
- Follow Up Care - Composite Rate
- Follow Up Care - Coordination of Care
- Follow Up Care - LVEF Assessment
- Follow Up Care - Pelvic Exam
- Follow-up Contact
- Follow-up Contact Date
- Gestational Age
- Glucose POD 1
- Glucose POD 2
- Goal Attainment
- Goal Setting
- Goals Collaborative
- Health Care Organization Identifier
- Health Care Staffing Services
- Health Care-Associated Bloodstream Infections in Newborns
- Health Status
- Healthcare Associated PN
- Heart Failure
- Hispanic Ethnicity
- Home Management Plan of Care (HMPC) Document Given to Patient/Caregiver
- Home Management Plan of Care Document Addresses Arrangements for Follow-up Care
- Home Management Plan of Care Document Addresses Environmental Control and Control of Other Triggers
- Home Management Plan of Care Document Addresses Methods and Timing of Rescue Actions
- Home Management Plan of Care Document Addresses Use of Controllers
- Home Management Plan of Care Document Addresses Use of Relievers
- Home Management Plan of Care Document Given to Patient/Caregiver
- Home Management Plan of Care Document Present
- Hospital Based Inpatient Psychiatric Services
- Hospital Outpatient ACEI or ARB Prescribed for LVSD
- Hospital Outpatient Activity Recommendations
- Hospital Outpatient Aldosterone Receptor Antagonists Prescribed for LVSD
- Hospital Outpatient Beta-Blocker Therapy (i.e., Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Succinate) Prescribed for LVSD
- Hospital Outpatient Cardiac Resynchronization Therapy (CRT, CRT with pacing CRT-P, CRT with defribrillator CRT-D)
- Hospital Outpatient Department
- Hospital Outpatient Discussion of Advance Directives
- Hospital Outpatient ICD Counseling for LVSD
- Hosptial Outpatient Assessment of Functional Status for Heart Failure
- Hours Worked
- Hours of physical restraint use
- Hours of seclusion use
- ICD Counseling
- ICD Population Size
- ICD-9-CM Diagnosis Code
- ICD-9-CM Other Diagnosis Codes
- ICD-9-CM Other Procedure Codes
- ICD-9-CM Other Procedure Dates
- ICD-9-CM Principal Diagnosis Code
- ICD-9-CM Principal Procedure Code
- ICD-9-CM Principal Procedure Date
- ICU Transfer or Admission Within First 24 Hours
- ICU VTE Prophylaxis
- IV Thrombolytic Therapy Administered
- Identified Pathogen
- Immunization
- Implanted Device
- In-Hospital LDL-Cholesterol Test
- Incidence of Potentially Preventable Hospital-Acquired VTE
- Infection Prior to Anesthesia
- Influenza Vaccination
- Influenza Vaccination Status
- Information Addressed Risks, Benefits and Alternatives to Transfusion
- Initial Antibiotic Received Within 4 Hours of Hospital Arrival
- Initial Antibiotic Received Within 6 Hours of Hospital Arrival (NQF-ENDORSED VOLUNTARY CONSENSUS STANDARDS FOR HOSPITAL CARE)
- Initial Antibiotic Received Within 8 Hours of Hospital Arrival - RETIRED
- Initial Antibiotic Selection for Community-Acquired Pneumonia (CAP) in Immunocompetent Patients
- Initial Antibiotic Selection for Community-Acquired Pneumonia (CAP) in Immunocompetent Patients – Intensive Care Unit (ICU) Patients
- Initial Antibiotic Selection for Community-Acquired Pneumonia (CAP) in Immunocompetent Patients – Non ICU Patients
- Initial Blood Culture Collection Date
- Initial Blood Culture Collection Time
- Initial ECG Interpretation
- Initial Patient Population Size – Medicare Only
- Initial Patient Population Size – Non-Medicare Only
- Initial Rhythm
- Initiation of Therapeutic Hypothermia
- Inpatient Mortality
- Inpatient Neonatal Mortality
- Instructions on Hand Washing
- Instructions to Contact Provider
- Intervention for Bone Health Risk
- Intervention for Distress
- Intervention for Distress
- Intervention for Fatigue
- Intervention for Fatigue
- Intervention for Lymphedema
- Intervention for Menopausal Symptoms
- Intervention for Neuropathy
- Intervention for Pain
- Intervention for Psychosocial Distress
- Intervention for Sleep
- Intervention for Sleep -Wake Disturbance
- Intervention for Sleep-Wake Disturbance
- Job Appropriate Credentials
- Joint Revision
- LDL ≥ 100 mg/dL
- LDL Cholesterol Assessment (Optional Test Measure)
- LVEF Assessment
- LVF Assessment
- LVSD
- LVSD < 40%
- LVSD of < 35%
- Laboratory Investigation for Secondary Causes of Osteoporosis
- Laboratory Testing Performed in the Prior 12 Months
- Laboratory Tests Ordered or Performed Prior to Discharge
- Laparoscope
- Last Name
- Left Bundle Branch Block (LBBB) on ECG
- Left Ventricular Assist, Device Biventricular Assist Device
- Lipid-Lowering Agent Prescribed at Discharge
- Lipid-Lowering Therapy at Discharge (Optional Test Measure)
- Maintenance of Thermoregulation in Therapeutic Hypothermia
- Measure Category Assignment
- Measure Category Assignment
- Measure Set
- Measurement Value
- Median Time from ED Arrival to ED Departure for Admitted ED Patients
- Median Time to ECG
- Median Time to Fibrinolysis
- Median Time to Fibrinolysis
- Median Time to Primary PCI
- Median Time to Transfer to Another Facility for Coronary Intervention
- Methods of Intubation Confirmation
- Minutes of Physical Restraint
- Minutes of Seclusion
- Multiple Antipsychotic Medications at Discharge with Appropriate Justification- Adolescent (13 through 17 years)
- Multiple Antipsychotic Medications at Discharge with Appropriate Justification- Adult (18 through 64 years)
- Multiple Antipsychotic Medications at Discharge with Appropriate Justification- Children (1 through 12 years)
- Multiple Antipsychotic Medications at Discharge with Appropriate Justification- Older Adult (≥ 65 years)
- Multiple Antipsychotic Medications at Discharge with Appropriate Justification- Overall Rate
- Multiple Antipsychotic Medications at Discharge- Adolescent (13 through 17 years)
- Multiple Antipsychotic Medications at Discharge- Adult (18 through 64 years)
- Multiple Antipsychotic Medications at Discharge- Children (1 through 12 years)
- Multiple Antipsychotic Medications at Discharge- Older Adult (≥ 65 years)
- Multiple Antipsychotic Medications at Discharge- Overall Rate
- Multiple Births
- Myelosuppressive Chemotherapy
- NPO (Nothing by Mouth) For Entire Hospital Stay
- NYHA Class
- National Provider Identifier
- Neuraxial Anesthesia
- Neutropenia Risk Cycle
- Newborn Admission Source
- Non-Primary PCI
- Number of Antipsychotic Medications Prescribed at Discharge
- Number of Cases with UTD Allowable Values
- Number of Rejected Cases
- Nursing Care Hours per Patient Day
- Nursing-Sensitive Care
- On FDA-approved Pharmacotherapy for Treatment of Osteoporosis Prior to Fracture
- Optimal Medication Management
- Oral Administration of Vitamin D
- Oral Antibiotics
- Osteoporosis
- Other Fracture Risk Assessment Method Ordered or Performed Prior to Discharge
- Other Surgeries
- Outpatient Encounter Date
- Parity
- Patient Ambulatory at End of Hospital Day Two
- Patient Blood Management
- Patient Discharged on Anticoagulation Therapy
- Patient Falls
- Patient HIC#
- Patient ID Verification
- Patient Identifier
- Patient Location
- Patient Referral to Next Level of Care Provider
- Patient Strengths
- Patients discharged on multiple antipsychotic medications
- Patients discharged on multiple antipsychotic medications with appropriate justification
- Patients with Atrial Fibrillation Receiving Anticoagulation Therapy
- Payment Source
- Pelvic Exam
- Performance Measure Identifier
- Performance Measurement System Identifier
- Perinatal Care
- Perioperative Death
- Personnel File Record Identifier
- Physical Restraint- Adolescent (13 through 17 years)
- Physical Restraint- Adult (18 through 64 years)
- Physical Restraint- Children (1 through 12 years)
- Physical Restraint- Older Adult (≥ 65 years)
- Physical Restraint- Overall Rate
- Physician 1
- Physician 2
- Plan for LDL-Cholesterol Test
- Plasma ID
- Plasma Event Total Doses
- Plasma Transfusion Indication
- Platelet ID
- Platelet Event Total Doses
- Platelet Transfusion Indication
- Pneumococcal Vaccination
- Pneumococcal Vaccination Status
- Pneumonia
- Pneumonia Diagnosis: ED/Direct Admit
- Point of Origin for Admission or Visit
- Point of Origin for Admission or Visit
- Point of Origin for Admission or Visit-Stroke
- Post Discharge Continuing Care Plan Transmitted - Adolescent (13 through 17 years)
- Post Discharge Continuing Care Plan Transmitted - Adult (18 through 64 years)
- Post Discharge Continuing Care Plan Transmitted - Children (1 through 12 years)
- Post Discharge Continuing Care Plan Transmitted - Older Adult (≥65 years)
- Post Discharge Continuing Care Plan Transmitted- Overall Rate
- Post Discharge Continuing Care Plan- Adolescent (13 through 17 years)
- Post Discharge Continuing Care Plan- Adult (18 through 64 years)
- Post Discharge Continuing Care Plan- Children (1 through 12 years)
- Post Discharge Continuing Care Plan- Older Adult (≥ 65 years)
- Post Discharge Continuing Care Plan- Overall Rate
- Post discharge continuing care plan created
- Post discharge continuing care plan transmitted to next level of care provider upon discharge
- Post-Discharge Appointment Scheduled Within 7 Days
- Post-Discharge Appointment for Heart Failure Patients
- Post-Discharge Evaluation Conducted Within 72 Hours
- Post-Discharge Evaluation for Heart Failure Patients
- Post-Treatment Education
- Post-Treatment Education - Community Resources
- Post-Treatment Education - Composite Rate
- Post-Treatment Education - Diet
- Post-Treatment Education - Exercise
- Post-Treatment Education - Late Effects
- Post-Treatment Education - Lymphedema
- Post-Treatment Education - Recurrence
- Postal Code
- Postoperative Infections
- Practice Environmental Scale-Nursing Work Index
- Pre-Arrival LDL-Cholesterol Qualitative Description
- Pre-Arrival LDL-Cholesterol Test
- Pre-Arrival LDL-Cholesterol Value
- Pre-Arrival Lipid-Lowering Agent
- Pre-Treatment Assessment
- Pre-Treatment Assessment - Distress
- Pre-Treatment Assessment - Fatigue
- Pre-Treatment Assessment - Overall Rate
- Pre-Treatment Assessment - Sleep-Wake Disturbance
- Pre-transfusion Hematocrit Result
- Pre-transfusion Hemoglobin
- Pre-transfusion Hemoglobin
- Pre-transfusion Hemoglobin Result
- Pre-transfusion INR Result
- Pre-transfusion Laboratory Testing
- Pre-transfusion PT/INR
- Pre-transfusion Platelet Count
- Pre-transfusion Platelet Count Result
- Pre-transfusion Platelet Testing
- Pre-transfusion Platelet Testing
- Preadmission Warfarin
- Predicted Value
- Pregnancy and Related Conditions
- Preoperative Anemia Screening
- Preoperative Anemia Screening
- Preoperative Anemia Screening Hematocrit Result
- Preoperative Anemia Screening Hemoglobin Result
- Preoperative Anemia Screening Result Date
- Preoperative Blood Type Testing
- Preoperative Blood Type Testing and Antibody Screening
- Preoperative Hair Removal
- Prescription for Alcohol or Drug Disorder Medication
- Prescription for Tobacco Cessation Medication
- Pressure Ulcer Prevalence
- Primary PCI Received Within 90 Minutes of Hospital Arrival
- Prior Diagnosis of Osteoporosis
- Prior Uterine Surgery
- Progress Toward Goals
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - CABG
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - Colon Surgery
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - Hip Arthroplasty
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - Hysterectomy
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - Knee Arthroplasty
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - Other Cardiac Surgery
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - Overall Rate
- Prophylactic Antibiotic Received Within One Hour Prior to Surgical Incision - Vascular Surgery
- Prophylactic Antibiotic Selection for Surgical Patients
- Prophylactic Antibiotic Selection for Surgical Patients - CABG
- Prophylactic Antibiotic Selection for Surgical Patients - Colon Surgery
- Prophylactic Antibiotic Selection for Surgical Patients - Hip Arthroplasty
- Prophylactic Antibiotic Selection for Surgical Patients - Hysterectomy
- Prophylactic Antibiotic Selection for Surgical Patients - Knee Arthroplasty
- Prophylactic Antibiotic Selection for Surgical Patients - Other Cardiac Surgery
- Prophylactic Antibiotic Selection for Surgical Patients - Overall Rate
- Prophylactic Antibiotic Selection for Surgical Patients - Vascular Surgery
- Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time
- Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time - Colon Surgery
- Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time - Hip Arthroplasty
- Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time - Knee Arthroplasty
- Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time - Overall Rate
- Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time - Vascular Surgery
- Prophylactic Antibiotics Discontinued Within 24 Hours After Surgery End Time – Hysterectomy
- Prophylactic Antibiotics Discontinued Within 48 Hours After Surgery End Time - Other Cardiac Surgery
- Prophylactic Antibiotics Discontinued Within 48 Hours After Surgery End Time – CABG
- Provider ID
- Provider ID
- Pseudomonas Risk
- Psychiatric Care Setting
- Psychiatric Inpatient Days - Medicare Only
- Psychiatric Inpatient Days-Non-Medicare Only
- Psychological Trauma History
- Psychosocial Distress Level Begin
- Psychosocial Distress Level End
- Pulse Oximetry Done
- RBC Event Total Doses
- RBC ID
- RBC Transfusion Indication
- RBC Unit Exclusions
- Race
- Re-Assessment for Distress
- Re-Assessment for Fatigue
- Re-Assessment for Sleep-Wake Disturbance
- Reason for Delay in Fibrinolytic Therapy
- Reason for Delay in PCI
- Reason for Early Discontinuation of Therapeutic Hypothermia
- Reason for No ACEI and No ARB prescribed for LVSD in Outpatient Setting
- Reason for No Activity Recommendations in the Outpatient Setting
- Reason for No Aldosterone Receptor Antagonist Prescribed for LVSD in the Outpatient Setting
- Reason for No Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Prescribed for LVSD at Discharge
- Reason for No Bisoprolol, Carvedilol, or Sustained-Release Metoprolol Prescribed for LVSD in the Outpatient Setting
- Reason for No Cardiac Resynchronization Therapy in the Outpatient Setting
- Reason for No DXA Scan
- Reason for No FDA-approved Pharmacotherapy for Treatment of Osteoporosis
- Reason for No ICD Counseling in the Outpatient Setting
- Reason for No LDL-Cholesterol Testing
- Reason for No Lipid-Lowering Therapy
- Reason for No Post-Discharge Appointment Within 7 Days
- Reason for No Preoperative Anemia Screening
- Reason for No Tobacco Cessation Medication During the Hospital Stay
- Reason for No Tobacco Cessation Medication at Discharge
- Reason for Not Administering Antenatal Steroid
- Reason for Not Administering Therapeutic Hypothermia
- Reason for Not Exclusively Feeding Breast Milk
- Reason for Not Prescribing CSF
- Reason for Not Recommending Exercise
- Reason for Transfer
- Reason for Transfer Out Prior to Therapeutic Hypothermia
- Referral for Addictions Treatment
- Referral for Outpatient Tobacco Cessation Counseling
- Relievers Administered
- Relievers for Inpatient Asthma
- Relievers for Inpatient Asthma (age 13 through 17 years)
- Relievers for Inpatient Asthma (age 2 through 17 years) – Overall Rate
- Relievers for Inpatient Asthma (age 2 through 4 years)
- Relievers for Inpatient Asthma (age 5 through 12 years)
- Report Period
- Restraint Prevalence (vest and limb)
- Return of Spontaneous Circulation
- Return of Spontaneous Circulation
- Risk Assessment/Treatment After Fracture
- Risk Factors for Drug-Resistant Pneumococcus
- Sample
- Sample Size – Medicare Only
- Sample Size – Non-Medicare Only
- Sampling Frequency
- Seclusion- Adolescent (13 through 17 years)
- Seclusion- Adult (18 through 64 years)
- Seclusion- Children (1 through 12 years)
- Seclusion- Older Adult (≥ 65 years)
- Seclusion- Overall Rate
- Sex
- Skill Mix
- Sleep-Wake Disturbance
- Smoking Cessation / Advice / Counseling
- Smoking Cessation Counseling for Acute Myocardial Infarction
- Smoking Cessation Counseling for Heart Failure
- Smoking Cessation Counseling for Pneumonia
- Specified Laboratory Tests
- Spontaneous Rupture of Membranes
- Stroke Education
- Stroke Performance Measures
- Substance Use
- Substance Use Measures
- Sudden Cardiac Arrest
- Surgery End Date
- Surgery End Time
- Surgery Patients Who Received Appropriate Venous Thromboembolism Prophylaxis Within 24 Hours Prior to Surgery to 24 Hours After Surgery
- Surgery Patients on Beta-Blocker Therapy Prior to Admission Who Received a Beta-Blocker During the Perioperative Period
- Surgery Patients with Appropriate Hair Removal
- Surgery Patients with Recommended Venous Thromboembolism Prophylaxis Ordered
- Surgery Scheduled Timeframe
- Surgery Start Date
- Surgical Care Improvement Project
- Surgical Incision Time
- Surgical Procedure
- Survivorship Visits
- Symptom - Bone Health Risk
- Symptom - Fatigue
- Symptom - Lymphedema
- Symptom - Menopausal
- Symptom - Neuropathy
- Symptom - Pain
- Symptom - Psychosocial Distress
- Symptom - Sleep
- Symptom Assessment
- Symptom Assessment - Bone Health Risk
- Symptom Assessment - Composite Rate
- Symptom Assessment - Fatigue
- Symptom Assessment - Lymphedema
- Symptom Assessment - Menopausal
- Symptom Assessment - Neuropathy
- Symptom Assessment - Pain
- Symptom Assessment - Psychosocial Distress
- Symptom Assessment - Sleep
- Symptom Intervention
- Symptom Intervention - Bone Health Risk
- Symptom Intervention - Composite Rate
- Symptom Intervention - Fatigue
- Symptom Intervention - Lymphedema
- Symptom Intervention - Menopausal
- Symptom Intervention - Neuropathy
- Symptom Intervention - Pain
- Symptom Intervention - Psychosocial Distress
- Symptom Intervention - Sleep
- Systemic Corticosteroids Administered
- Systemic Corticosteroids for Inpatient Asthma
- Systemic Corticosteroids for Inpatient Asthma (age 13 through 17 years)
- Systemic Corticosteroids for Inpatient Asthma (age 2 through 17 years) – Overall Rate
- Systemic Corticosteroids for Inpatient Asthma (age 2 through 4 years)
- Systemic Corticosteroids for Inpatient Asthma (age 5 through 12 years)
- Temperature Value
- Therapeutic Hypothermia Initiated
- Thermoregulation Maintained
- Third or Fourth Degree Laceration
- Thrombolytic Therapy Administered
- Time IV Thrombolytic Therapy Administered at This Hospital
- Time Last Known Well
- Time Therapeutic Hypothermia Ended
- Time Therapeutic Hypothermia Initiated
- Time Therapeutic Hypothermia Ordered
- Timeliness of First Defibrillation Attempt
- Timely Confirmation of Correct Endotracheal Tube
- Tobacco Treatment Measures
- Tobacco Use Screening
- Tobacco Use Status
- Tobacco Use Status Post-Discharge (Not used in measure calculation)
- Tobacco Use Treatment
- Tobacco Use Treatment
- Tobacco Use Treatment FDA-Approved Cessation Medication
- Tobacco Use Treatment Practical Counseling
- Tobacco Use Treatment Provided or Offered
- Tobacco Use Treatment Provided or Offered
- Tobacco Use Treatment Provided or Offered at Discharge
- Tobacco Use Treatment Provided or Offered at Discharge
- Tobacco Use Treatment at Discharge
- Tobacco Use: Assessing Status after Discharge
- Total Leave Days - Medicare Only
- Total Leave Days-Non-Medicare Only
- Transfer From Another ED
- Transferred From or Expired in Emergency Department
- Transfusion Consent
- Transfusion Consent
- Transfusion Order
- Transfusion Start Date
- Transfusion Start Time
- Transfusion Type
- Treatment - Bilateral Mastectomy
- Treatment - Bone Health Risk
- Treatment - Breast Conserving
- Treatment - Menopause Inducer
- Treatment - Neuropathy Causing
- Treatment - Surgery or Radiation
- Treatment - Tamoxifen
- Treatment - Trastuzumab
- Underlying Cause of Osteoporosis
- Unique Blinded Case Identifier
- Unique Blinded Health Care Organization Identifier
- Urinary Catheter-Associated Urinary Tract Infection Rate
- VBAC
- VTE Discharge Instructions
- VTE Patients Receiving UFH with Dosages/Platelet Count Monitoring by Protocol or Nomogram
- VTE Patients with Anticoagulation Overlap Therapy
- VTE Prophylaxis
- VTE Prophylaxis
- VTE Timely
- Vancomycin
- Vendor Tracking Identifier
- Venous Thromboembolism
- Venous Thromboembolism (VTE) Prophylaxis
- Ventilator-Associated Pneumonia Rate
- Violence Risk to Others
- Violence Risk to Self
- Vital Sign Monitoring
- Voluntary Turnover
- Written Discharge Instructions
- deleted from ACHF - Care Transition Record-Condition or Functional Status at Discharge
Risk Adjustment: No.
Data Collection Approach: Retrospective data sources for required data elements include administrative/billing data and medical records.
Data Accuracy: Hospitals may wish to implement periodic audits to monitor and ensure data accuracy.
Measure Analysis Suggestions: In order to further examine the issue of restraint use within a facility it may be useful to study the incidence of physical restraint use by collecting additional information about the clinical justification for use.
Sampling: No.
Data Reported As: Aggregate rate generated from count data reported as a ratio .
Selected References:
- Donat, D. (August, 2003). An analysis of successful efforts to reduce the use of seclusion and restraint at a public psychiatric hospital. Psychiatric Services. 54(8): 1119-1123.
- Fisher, W. A. (2003). Elements of successful restraint and seclusion reduction programs and their application in a large, urban, state psychiatric hospital. Journal of Psychiatric Practice, 9(1), 7-15.
- Huckshorn, K.A. (2004/September). Reducing seclusion and restraint use in mental health settings: Core strategies for prevention. Journal of Psychosocial Nursing and Mental Health Services. 42(9). Pp. 22-31.
- Mohr, W. K., & Anderson, J. A. (2001). Faulty assumptions associated with the use of restraints with children. Journal of Child and Adolescent Psychiatric Nursing, 14(3), 141- 151.
- Special Section on Seclusion and Restraint, (2005, Sept). Psychiatric Services, 56 (9), 1104-1142.
- Success Stories and Ideas for Reducing Restraint/Seclusion. (2003). A compendium of strategies created by the American Psychiatric Association (APA), the American Psychiatric Nurses Association (APNA), the National Association of Psychiatric Health Systems (NAPHS), and the American Hospital Association Section for Psychiatric and Substance Abuse Services (AHA). Retrieved from the Internet on February 10, 2010 at http://www.naphs.org
Measure Algorithm: