Measure Information Form
Version 2025A
Measure Information Form
Included Populations: Not applicable Excluded Populations: None Data Elements:Denominator Statement: Heart failure patients with current or prior documentation of left ventricular ejection fraction (LVSD) ≤40%.
Included Populations:Excluded Populations:
- An ICD-10-CM Principal Diagnosis Code for HF as defined in Appendix A, Table 2.1
- Documentation of LVSD ≤40%
Data Elements:
- Patients less than 18 years of age
- Patients with a documented Reason for No Mineralocorticoid Receptor Antagonist Prescribed at Discharge
- Patients who expired
- Patients who left against medical advice Patients who left against medical advice (AMA)
- Patients discharged to another hospital
- Patients discharged to home for hospice care
- Patients discharged to a healthcare facility for hospice care
- Patients who have a Length of Stay greater than 120 days
- Patients with Comfort Measures Only documented
- Patients enrolled in a Clinical Trial
- Patients who had a left ventricular assist device (LVAD) or heart transplant procedure (ICD-10-PCS Procedure Code for LVAD or heart transplant as defined in Appendix A, Table 2.2 or Table 2.13)
- Admission Date
- Birthdate
- Clinical Trial
- Comfort Measures Only
- Discharge Date
- Discharge Disposition
- ICD-10-CM Other Diagnosis Codes
- ICD-10-CM Principal Diagnosis Code
- ICD-10-PCS Other Procedure Codes
- ICD-10-PCS Other Procedure Dates
- ICD-10-PCS Principal Procedure Code
- ICD-10-PCS Principal Procedure Date
- LVSD
- Reason for No Mineralocorticoid Receptor Antagonist (MRA) Prescribed at Discharge
- American Heart Association. Get With The Guidelines® Heart Failure Fact Sheet. 2016.
- Heidenreich, P., Bozkurt, B., Aguilar, D., Allen, L., Byun, J., Colvin, M., Deswal, A., Drazner, M., Dunlay, S., Evers, L., Fang, J., Fedson, S., Fonarow, G., Hayek, S., Hernandez, A., Khazanie, P., Kittleson, M., Lee, C., Link, M., Milano, C., Nnacheta, L., Sandhu, A., Stevenson, L., Vardeny, O., Vest, A., & Yancy, C. 2022 AHA/ACC2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032.
- Hunt SA, Abraham WT, Chin MH, Felman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated Into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
- Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Klapholz M, MoserDK, Rogers JG, Starling RC, Stevenson WG, Tang WHW, Teerlink JR, Walsh MN. Executive Summary: HFSA 2010 Comphrensive Heart Failure Practice Guideline. J Card Fail 2010;16:475-539.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327.
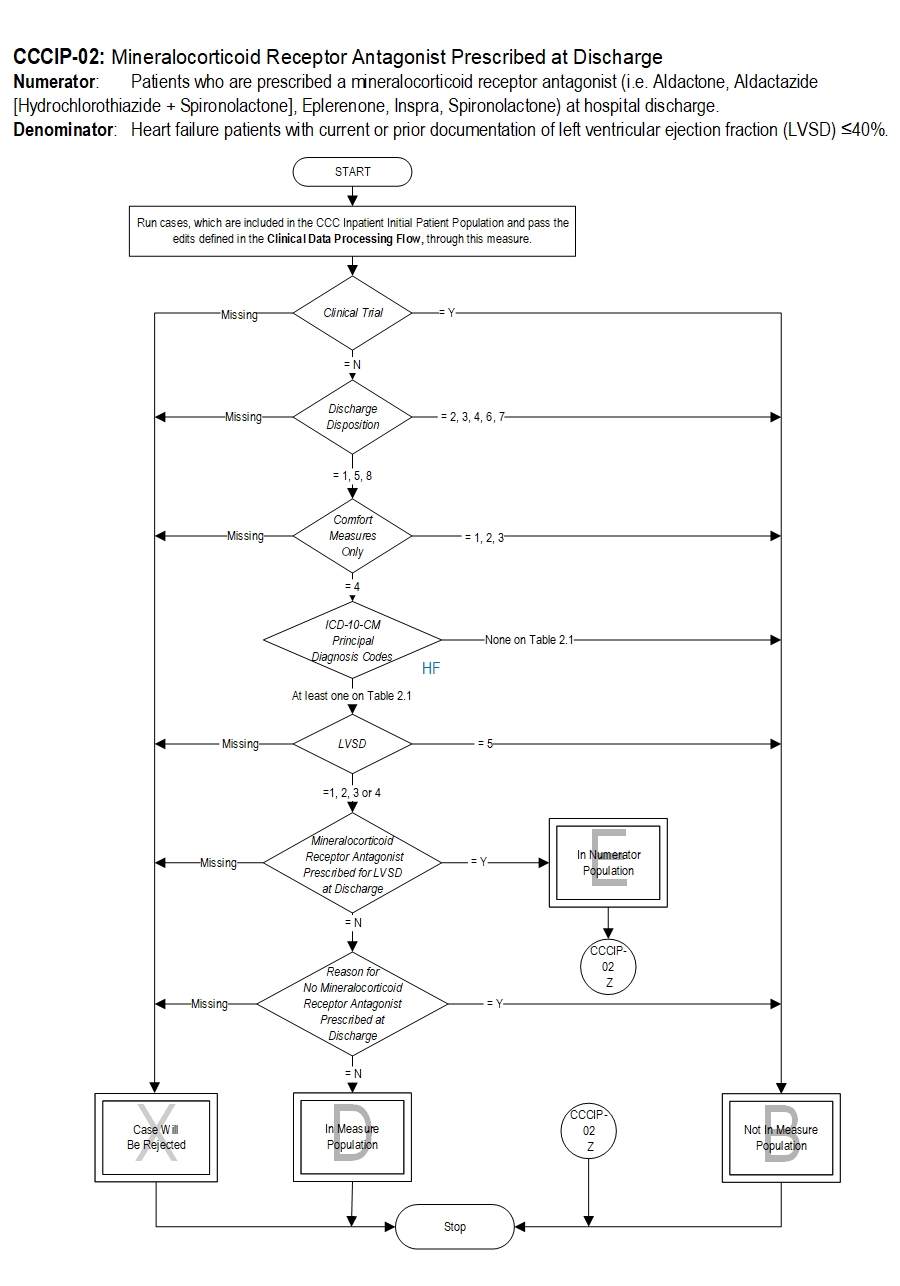
CCCIP-02 Mineralocorticoid Receptor Antagonist (MRA) Prescribed at Discharge Algorithm Narrative
Numerator: Patients who are prescribed an mineralocorticoid receptor antagonist (i.e. Aldactone, Aldactazide [Hydrochlorothiazide + Spironolactone], Eplerenone, Inspra, Spironolactone) at hospital discharge.Denominator: Heart failure patients with current or prior documentation of left ventricular ejection fraction (LVSD) less than or equal to 40%.
1. Start processing. Run cases, which are included in the CCC Inpatient Initial Patient Population and pass the edits defined in the Clinical Data Processing Flow, through this measure.
2. Check Clinical Trial.
- If Clinical Trial is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Clinical Trial equals Y the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Clinical Trial equals N continue processing and proceed to check Discharge Disposition.
- If Discharge Disposition is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Discharge Disposition equals 2, 3, 4, 6 or 7 the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Discharge Disposition equals 1, 5 or 8 continue processing and proceed to check Comfort Measures Only.
- If Comfort Measures Only is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Comfort Measures Only equals 1, 2 or 3 the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Comfort Measures Only equals 4 continue processing and proceed to check ICD-10-CM Principal Diagnosis Codes.
- If ICD-10-CM Principal Diagnosis Codes have None on Table 2.1 the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If ICD-10-CM Principal Diagnosis Codes have at least one on Table 2.1 continue processing and proceed to check LVSD.
- If LVSD is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If LVSD equals 5 the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If LVSD equals 1, 2, 3 or 4 continue processing and proceed to check Mineralocorticoid Receptor Antagonist (MRA) Prescribed for LVSD at Discharge.
- If Mineralocorticoid Receptor Antagonist (MRA) Prescribed for LVSD at Discharge is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Mineralocorticoid Receptor Antagonist (MRA) Prescribed for LVSD at Discharge equals Y the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population. Stop processing.
- If Mineralocorticoid Receptor Antagonist (MRA) Prescribed for LVSD at Discharge equals N continue processing and proceed to check Reason for No Mineralocorticoid Receptor Antagonist (MRA) Prescribed at Discharge.
- If Reason for No Mineralocorticoid Receptor Antagonist (MRA) Prescribed at Discharge is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Reason for No Mineralocorticoid Receptor Antagonist (MRA) Prescribed at Discharge equals Y the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Reason for No Mineralocorticoid Receptor Antagonist (MRA) Prescribed at Discharge equals N the case will proceed to a Measure Category Assignment of D and will be in the Measure Population. Stop processing.
CPT® only copyright 2024 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.