Measure Information Form
Version 2025A
Measure Information Form
**SUSPENDED for Thrombectomy-Capable Stroke Centers, Effective July 1, 2022**Included Populations: As above Excluded Populations: None Data Elements:Denominator Statement: Ischemic stroke patients treated with IV or IA alteplase therapy or who undergo mechanical endovascular reperfusion therapy
Included Populations:Excluded Populations:
- Discharges with ICD-10-CM Principal Diagnosis Code for ischemic stroke as defined in Appendix A, Table 8.1 for ICD-10 codes, AND
- Patients with documented thrombolytic (IV or IA alteplase) therapy (ICD-10-PCS Principal or Other Procedure Codes as defined in Appendix A, Table 8.1a for ICD-10 codes), OR
- Patients with documented Mechanical Endovascular Reperfusion Therapy (ICD-10-PCS Principal or Other Procedure Codes as defined in Appendix A, Table 8.1b for ICD-10 codes)
Data Elements:
- Patients less than 18 years of age
- Patients who have a Length of Stay > 120 days
- Patients admitted for Elective Carotid Intervention
- Patients who expire during the hospital stay
- Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks E. Guidelines for the Early Management of Adults with Ischemic Stroke: A Guideline From the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Stroke. 2007;38:1675-1678.
- Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007:38:2262-2269.
- Bruno A, Shah N, Lin C, Close B, Hess DC, Davis K, Baute V, Switzer JA, Waller JL, Nichols FT. Simplified modified Rankin scale questionnaire: reproducibility over the telephone and validation with quality of life. Stroke. 2011;42:2276-2279.
- Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et. al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. NEJM. 2015 Mar;372(11): 1009-17.
- Demchuk AM, Goyal M, Monon BK, Eesa M, Ryckborst KJ, Kamal N, et. al. Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times (ESCAPE) trial: methodology. Int J Stroke. 2015 Apr;10(3): 429-38.
- Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:32-36.
- Leifer D, Bravata DM, Connors JJ III, Hinchey JA, Jauch EC, Johnston SC, Latchaw R, Likosky W, Ogilvy C, Qureshi AI, Summers D, Sung GY, Williams LS, Zorowitz R, on behalf of the American Heart Association Special Writing Group of the Stroke Council, Atherosclerotic Peripheral Vascular Disease Working Group and Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular Nursing. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow-up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:857.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, et al; on behalf of the American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018 Jan;49:e10-e11.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke. A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418.
- Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin scale. Stroke. 2007:38:e144.
- Rankin J. Cerebral vascular accidents in patients over the age of 60. Scott Med J. 1957;2(5):200-15.
- Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et. al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. NEJM. 2015 Apr: 1-11.
- Schwamm LH, Holloway RG, Amarenco P. Audebert HJ, Bakas T, Chumbler NR, Handschu R, Jauch EC, Knight WA IV, Levine SR, Mayberg M, Meyer BC, Meyers PM, Skalabrin E, Wechsler LR; American Heart Association Stroke Council; Interdisciplinary Council on Peripheral Vascular Disease. A review of the evidence for the use of telemedicine within stroke systems of care: a scientific statement for the American Heart Association/American Stroke Association. Stroke. 2009;40:2616-2634.
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. New England Journal of Medicine 1995;333:1581-1587.
- Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, et. al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014 May;694): 260-4.
- Wilson JT, Hareendran A, Hendry A, Potter J. Bone I, Muir KW. Reliability of the modified Rankin scale across multiple raters: benefits of a structured interview. Stroke. 2005;36:777-781.
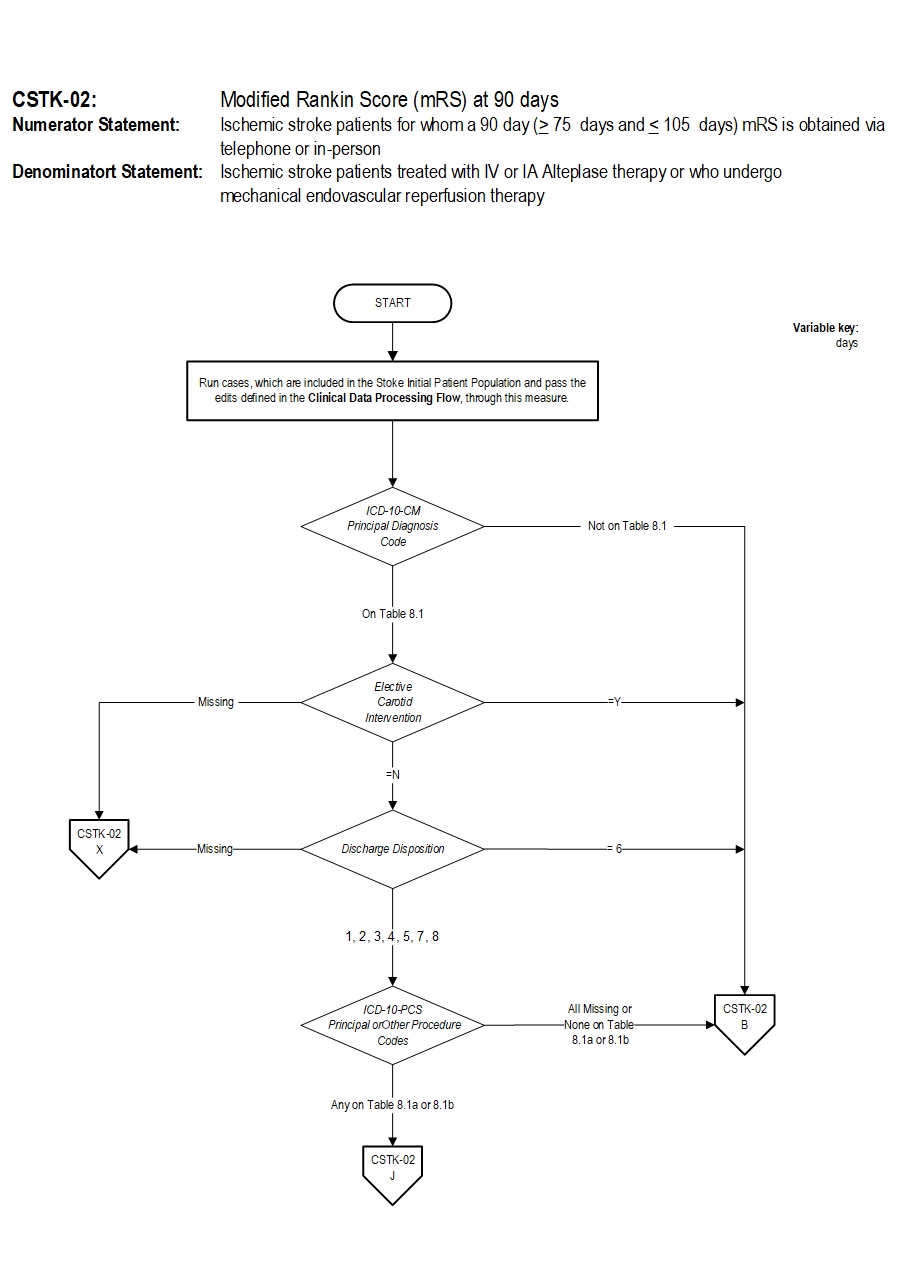
CSTK-02: Modified Rankin Score (mRS) at 90 days Algorithm Narrative
Numerator: Ischemic stroke patients for whom a 90 day (greater than or equal to 75 days and less than or equal to 105 days) mRS is obtained via telephone or in-person.Denominator: Ischemic stroke patients treated with IV or IA alteplase therapy or who undergo mechanical endovascular reperfusion therapy.
Variable Key: Days 1. Start processing. Run cases, which are included in the Comprehensive Stroke Initial Patient Population and pass the edits defined in the Clinical Data Processing Flow, through this measure.
2. Check ICD-10 CM Principal Diagnosis Code
- If all ICD-10 CM Principal Diagnosis Code are not on Table 8.1, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If at least one of the ICD-10 CM Principal Diagnosis Code is on Table 8.1, continue processing and proceed to check Elective Carotid Intervention.
- If Elective Carotid Intervention is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Elective Carotid Intervention equals Y the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Elective Carotid Intervention equals N continue processing and proceed to check Discharge Disposition.
- If Discharge Disposition is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Discharge Disposition equals 6 the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Discharge Disposition equals 1,2,3,4,5,7 or 8 continue processing and proceed to check ICD-10-PCS Principal or Other Procedure Codes
- If ICD-10-PCS Principal or Other Procedure Codes equals All Missing or None on Table 8.1a or 8.1b, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If ICD-10-PCS Principal or Other Procedure Codes equals Any on Table 8.1a or 8.1b continue processing and proceed to check Modified Rankin Score (mRS).
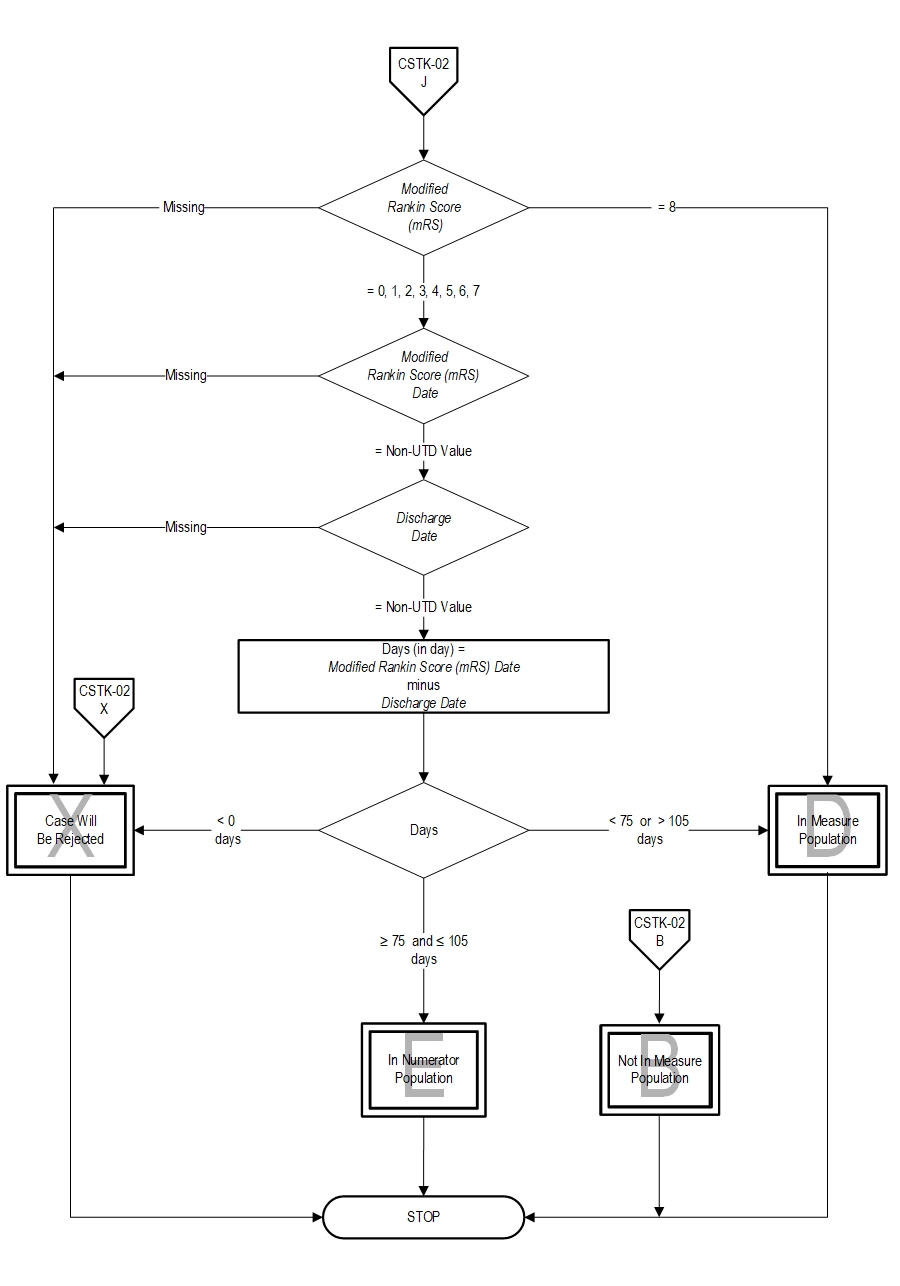
- If Modified Rankin Score (mRS) is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Modified Rankin Score (mRS) equals 8, the case will proceed to a Measure Category Assignment of D and will be in the Measure Population. Stop processing.
- If Modified Rankin Score (mRS) equals 0,1,2,3,4,5,6,or 7 continue processing and the case will proceed to a check Modified Rankin Score (mRS) Date.
- If Modified Rankin Score (mRS) Date is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Modified Rankin Score (mRS) Date equals Non unable to determine (UTD) value, continue processing and proceed to Discharge Date.
- If Discharge Date is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Discharge Date equals Non unable to determine (UTD) value, continue processing and proceed to the calculation of the Days.
- Continue processing and proceed to check Days.
- If Days is less than zero, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Days is less than 75 or greater than 105 days, the case will proceed to a Measure Category Assignment of D and will be in the Denominator Population. Stop processing.
- If Days is greater than or equal to 75 and less than or equal to 105 days the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population. Stop processing.
CPT® only copyright 2024 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.