Measure Information Form
Version 2024A1
Measure Information Form
** THIS MEASURE IS RETIRED AS OF 1/1/2024 FOR ACCREDITATION PROGRAMS**| Set Measure ID | Performance Measure Name |
|---|---|
| TOB-2 |
Tobacco Use Treatment Provided or Offered |
| TOB-2a |
Tobacco Use Treatment |
TOB-2 Patients identified as tobacco product users who receive or refuse practical counseling to quit AND receive or refuse FDA-approved cessation medications during the hospital stay.
TOB-2a Patients who received counseling AND medication as well as those who received counseling and had reason for not receiving the medication during the hospital stay.
The measure is reported as an overall rate which includes all patients to whom tobacco use treatment was provided, or offered and refused, and a second rate, a subset of the first, which includes only those patients who received tobacco use treatment. The Provided or Offered rate (TOB-2), describes patients identified as tobacco product users who receive or refuse practical counseling to quit AND receive or refuse FDA-approved cessation medications during the hospital stay. The Tobacco Use Treatment (TOB-2a) rate describes only those who received counseling AND medication as well as those who received counseling and had reason for not receiving the medication. Those who refused are not included.
Rationale: Tobacco use is the single greatest cause of disease in the United States today and accounts for more than 480,000 deaths each year (CDC MMWR, 2014). Smoking is a known cause of multiple cancers, heart disease, stroke, complications of pregnancy, chronic obstructive pulmonary disease, other respiratory problems, poorer wound healing, and many other diseases (CDC, 2020). Tobacco use creates a heavy cost to society as well as to individuals. Smoking-attributable health care expenditures are estimated to be at least $240 billion per year in direct medical expenses for adults, and over $185 billion in lost productivity (CDC, 2022).There is strong and consistent evidence that tobacco dependence interventions, if delivered in a timely and effective manner, significantly reduce the user's risk of suffering from tobacco-related disease and improve outcomes for those already suffering from a tobacco-related disease (CDC, 2021, DHHS, 2020, Choi et al, 2021, DHHS, 2000; Baumeister, 2007; Lightwood, 2003 and 1997; Rigotti, 2012). Effective, evidence-based tobacco dependence interventions have been clearly identified and include brief clinician advice, individual, group, or telephone counseling, and use of FDA-approved medications. These treatments are clinically effective and extremely cost-effective relative to other commonly used disease prevention interventions and medical treatments. Hospitalization (both because hospitals are a tobacco-free environment and because patients may be more motivated to quit as a result of their illness) offers an ideal opportunity to provide cessation assistance that may promote the patient's medical recovery.
Type Of Measure: Process Improvement Noted As: Increase in the rateTOB-2: The number of patients who received or refused practical counseling to quit AND received or refused FDA-approved cessation medications during the hospital stay. TOB-2a: The number of patients who received practical counseling to quit AND received FDA-approved cessation medications during the hospital stay.
Included Populations: TOB-2:Denominator Statement:TOB-2a:
- Patients who refuse counseling
- Patients who refuse FDA- Approved cessation medication
Excluded Populations: TOB-2 and TOB-2a
- Not applicable
For FDA Approved Medications OnlyData Elements:
- Smokeless tobacco users
- Pregnant smokers
- Patients with reasons for not administering FDA-approved cessation medication.
- TOB-2: The number of hospitalized inpatients 18 years of age and older identified as current tobacco users.
- TOB-2a: The number of hospitalized inpatients 18 years of age and older identified as current tobacco users excluding those whose tobacco use status is unknown.
Included Populations: Not applicable Excluded Populations:Data Elements:
- Patients less than 18 years of age
- Patient who are cognitively impaired
- Patients who are not current tobacco users
- Patients who refused screening for Tobacco Use Status during the hospital stay
- Patients who have a duration of stay less than or equal to one day or greater than 120 days
- Patients with Comfort Measures Only documented
Variation may exist in the assignment of ICD-10 codes; therefore, coding practices may require evaluation to ensure consistency.
Measure Analysis Suggestions: Hospitals may wish to identify those patients that refused either counseling or medications or both so as to have a better understanding of which treatment type is refused so that efforts can be directed toward improving care. Sampling: Yes. Please refer to the measure set specific sampling requirements and for additional information see the Population and Sampling Specifications section. Data Reported As: Aggregate rate generated from count data reported as a proportion. Selected References:- Baumeister, S. E., Schumann, A., Meyer, C., John, U., Volzke, H., & Alte, D. (2007). Effects of smoking cessation on health care use: Is elevated risk of hospitalization among former smokers attributable to smoking-related morbidity? Drug and Alcohol Dependence, 88(2–3), 197–203.
- Centers for Disease Control. (2022). Smoking & Tobacco Use: Costs and Expenditures. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/cost-and-expenditures.html.
- Centers for Disease Control and Prevention. (2021). Smoking & Tobacco Use: Clinical Interventions to Treat Tobacco Use and Dependence Among Adults. https://www.cdc.gov/tobacco/patient-care/care-settings/clinical/index.html
- Centers for Disease Control and Prevention. (2020). Smoking & Tobacco Use: Health Effects. https://www.cdc.gov/tobacco/basic_information/health_effects/index.htm
- Centers for Disease Control and Prevention. (2014). Current cigarette smoking among adults—United States, 2005–2013. Morbidity and Mortality Weekly Report (MMWR), 63(47), 1108–1112. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6347a4.htm?s_cid=mm6347a4_w.
- Choi, H. K., Ataucuri-Vargas, J., Lin, C., Singrey, A. (2021). The current state of tobacco cessation treatment. Cleveland Clinic Journal of Medicine, July 2021, 88(7), 393-404.
- Lightwood, J. M. (2003). The economics of smoking and cardiovascular disease. Progress in Cardiovascular Diseases, 46(1), 39–78.
- Lightwood, J. M., & Glantz, S. A. (1997). Short-term economic and health benefits of smoking cessation: Myocardial infarction and stroke. Circulation, 96(4), 1089–1096.
- Rigotti, N. A., Clair, C., Munafo, M. R., & Stead, L. F. (2012). Interventions for smoking cessation in hospitalised patients. Cochrane Database of Systematic Reviews. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22592676.
- U.S. Department of Health and Human Services. (2000). Reducing tobacco use: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention.
- United States Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking Cessation: A Report of the Surgeon General [Internet]. Washington (DC): US Department of Health and Human Services; 2020. Chapter 6, Interventions for Smoking Cessation and Treatments for Nicotine Dependence. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555596/
Algorithm Narrative
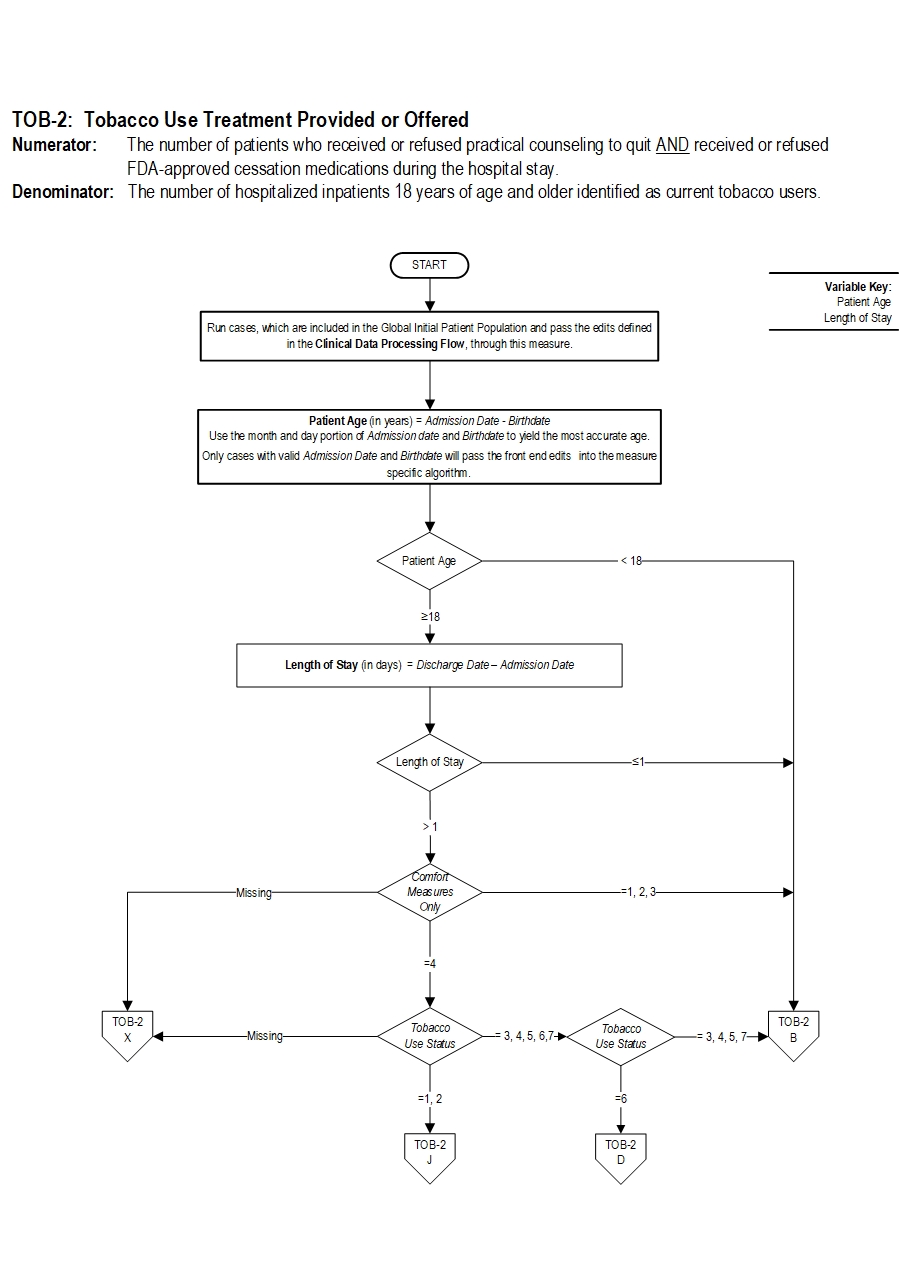
TOB-2: Tobacco Use Treatment Provided or OfferedNumerator: The number of patients who received or refused practical counseling to quit AND received or refused FDA-approved cessation medications during the hospital stay.
Denominator: The number of hospitalized inpatients 18 years of age and older identified as current tobacco users.
Variable key:
Patient Age
Length of Stay
1. Start processing. Run cases, which are included in the Global Initial Patient Population and pass the edits defined in the Clinical Data Processing Flow, through this measure.
2. Calculate Patient Age. Patient Age, in years, is equal to the Admission Date minus the Birthdate. Use the month and day portion of Admission Date and Birthdate to yield the most accurate age. Only cases with valid Admission Date and Birthdate will pass the front end edits into the measure specific algorithms.
3. Check Patient Age
a. If Patient Age is less than 18 years, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Patient Age is equal to or greater than 18 years, continue processing and proceed to calculate Length of Stay.
4. Calculate Length of Stay. Length of Stay, in days, is equal to the Discharge Date minus the Admission Date.
5. Check Length of Stay
a. If Length of Stay is equal to or less than 1 day, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Length of Stay is greater than 1 day, continue processing and proceed to check Comfort Measures Only.
6. Check Comfort Measures Only
a. If Comfort Measures Only is missing, the case will proceed to a Measure Category Assignment of X and will be rejected for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Comfort Measures Only is equal to 1, 2, or 3, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
c. If Comfort Measures Only is equal to 4, continue processing and proceed to check Tobacco Use Status.
7. Check Tobacco Use Status
a. If Tobacco Use Status is missing, the case will proceed to a Measure Category Assignment of X and will be rejected for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Tobacco Use Status equals 1 or 2, continue processing and proceed to step 9 to check Tobacco Use Treatment Practical Counseling.
c. If Tobacco Use Status equals 3, 4, 5, 6 or 7, continue processing and proceed to Step 8 to check Tobacco Use Status.
8. Check Tobacco Use Status
a. If Tobacco Use Status equals 3, 4, 5, or 7, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Tobacco Use Status equals 6, the case will proceed to a Measure Category Assignment of D and will be in the Measure Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
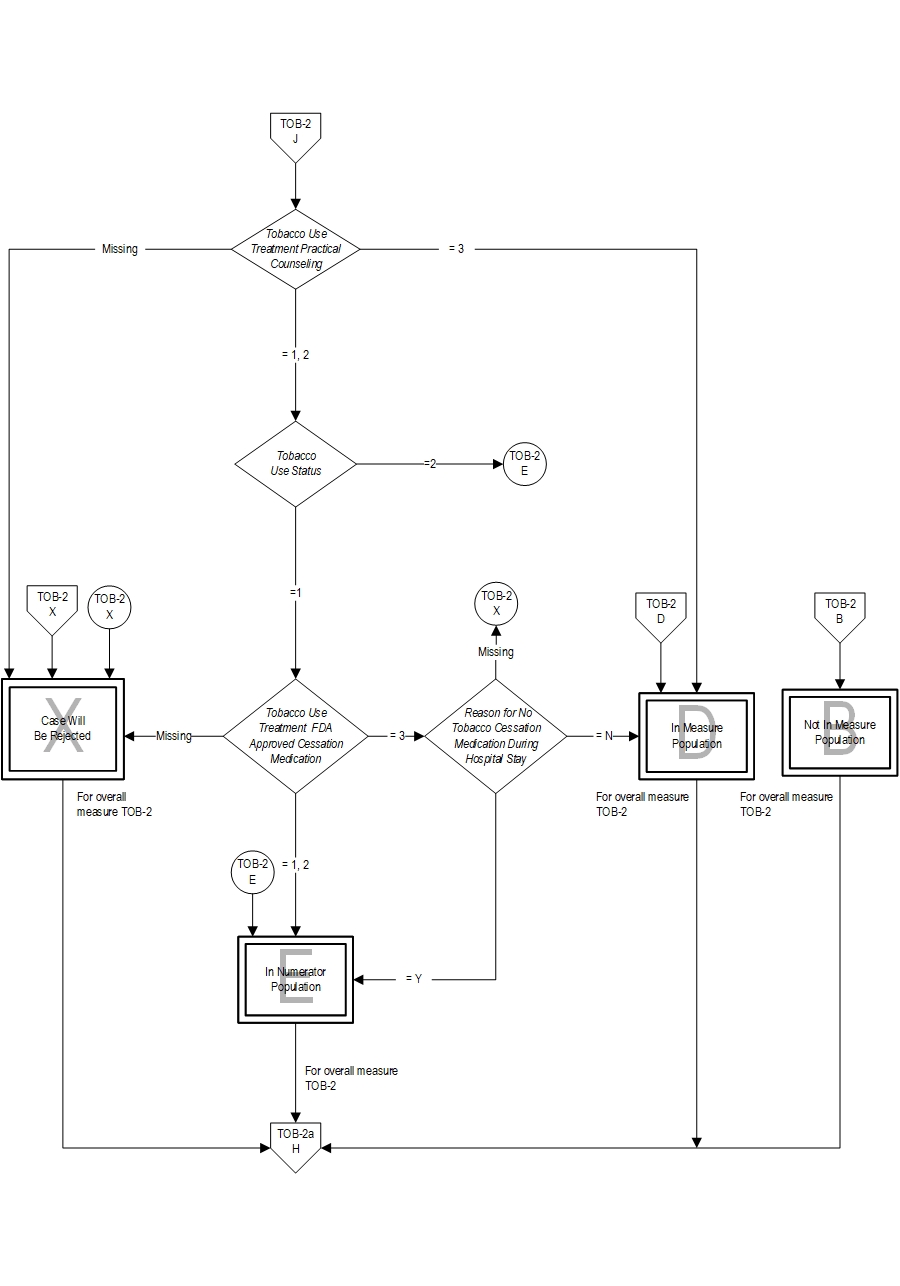
9. Check Tobacco Use Treatment Practical Counseling
a. If Tobacco Use Treatment Practical Counseling is missing, the case will proceed to a Measure Category Assignment of X and will be rejected for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Tobacco Use Treatment Practical Counseling equals 3, the case will proceed to Measure Category Assignment of D and will be in the Measure Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB 2a.
c. If Tobacco Use Treatment Practical Counseling equals 1 or 2, continue processing and proceed to Recheck Tobacco Use Status.
10. Recheck Tobacco Use Status
a. If Tobacco Use Status equals 2, the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB 2a.
b. If Tobacco Use Status equals 1, continue processing and proceed to Tobacco Use Treatment FDA-Approved Cessation Medication.
11. Check Tobacco Use Treatment FDA-Approved Cessation Medication
a. If Tobacco Use Treatment FDA-Approved Cessation Medication is missing, the case will proceed to a Measure Category Assignment of X and will be rejected for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Tobacco Use Treatment FDA-Approved Cessation Medication equals 3, continue processing and proceed to Reason for No Tobacco Cessation Medication During the Hospital Stay.
c. If Tobacco Use Treatment FDA-Approved Cessation Medication equals 1 or 2, the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB 2a.
12. Check Reason for No Tobacco Cessation Medication During Hospital Stay
a. If Reason for No Tobacco Cessation Medication During Hospital Stay is missing, the case will proceed to a Measure Category Assignment of X and will be rejected for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
b. If Reason for No Tobacco Cessation Medication During Hospital Stay equals N, the case will proceed to a Measure Category Assignment of D and will be in the Measure Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB-2a.
c. If Reason for No Tobacco Cessation Medication During Hospital Stay equals Y, the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population for the overall measure rate TOB-2. Continue processing and proceed to Step 13 to Initialize Measure Category Assignment for sub-measure TOB 2a.
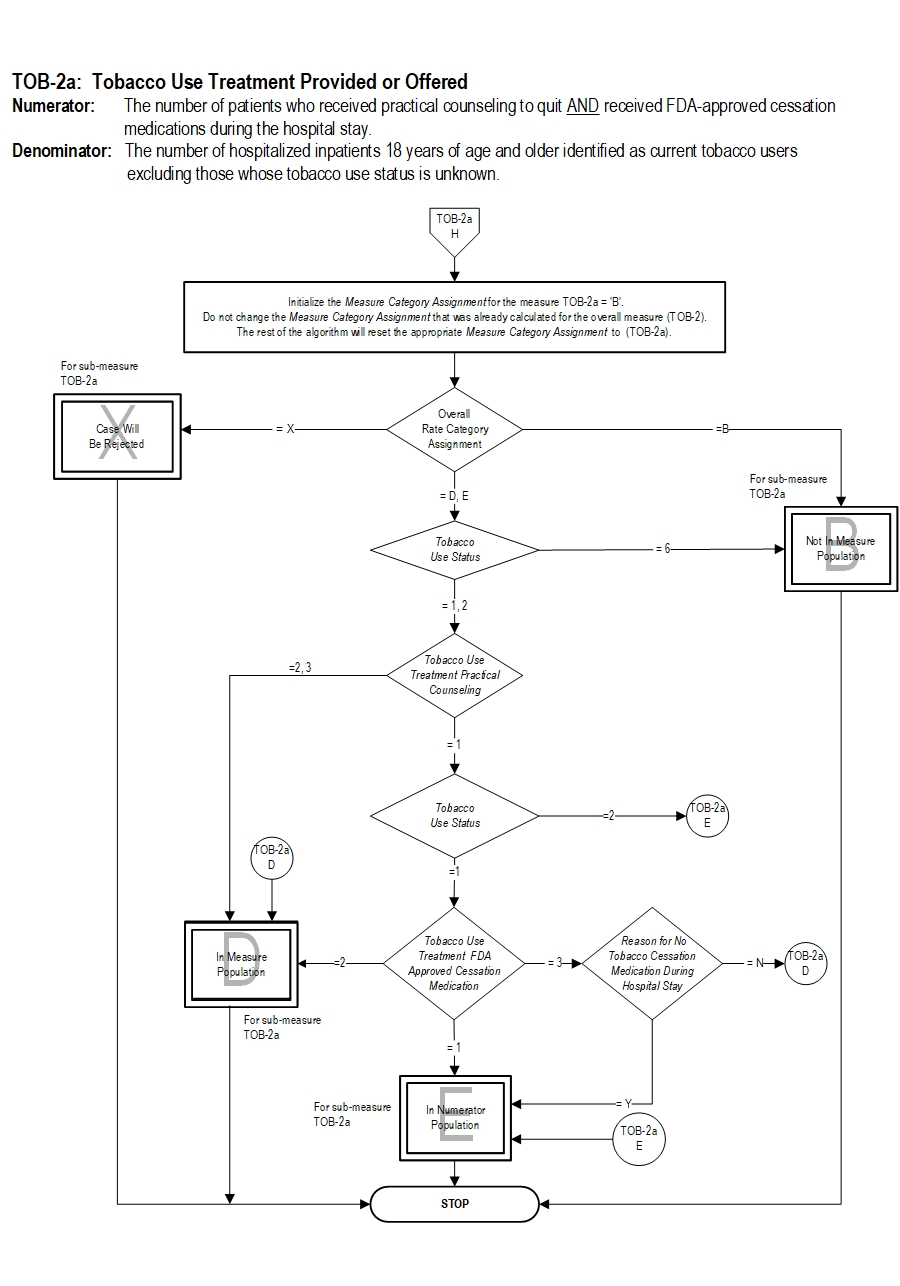
Algorithm Narrative
TOB-2a: Tobacco Use Treatment Numerator: The number of patients who received practical counseling to quit AND received FDA-approved cessation medications during the hospital stay.
Denominator: The number of hospitalized inpatients 18 years of age and older identified as current tobacco users excluding those whose tobacco use status is unknown.
13. Initialize Measure Category Assignment for sub-measure TOB-2a to Measure Category Assignment of B. Do not change the Measure Category Assignment that was already calculated for the overall measure TOB-2. The rest of the algorithm will reset the appropriate Measure Category Assignment to TOB-2a.
14. Check Overall Rate Category Assignment
a. If Overall Rate Category Assignment equals X, the case will proceed to a Measure Category Assignment of X and will not be in the Measure Population for sub-measure TOB-2a. Stop processing.
b. If the Overall Rate Category Assignment equals B, the case will proceed to Measure Category Assignment of B and will not be in the Measure Population for sub-measure TOB-2a. Stop processing.
c. If Overall Rate Category Assignment equals D or E, continue processing and proceed to recheck Tobacco Use Status.
15. Recheck Tobacco Use Status
a. If Tobacco Use Status equals 6, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population for sub-measure TOB-2a. Stop processing.
b. If Tobacco Use Status equals 1 or 2, continue processing and proceed to recheck Tobacco Use Treatment Practical Counseling.
16. Recheck Tobacco Use Treatment Practical Counseling
a. If Tobacco Use Treatment Practical Counseling equals 2 or 3, the case will proceed to Measure Category Assignment of D and will be in the Measure Population for sub-measure TOB-2a. Stop processing.
b. If Tobacco Use Treatment Practical Counseling equals 1, continue processing and proceed to recheck Tobacco Use Status.
17. Recheck Tobacco Use Status
a. If Tobacco Use Status equals 2, the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population for sub-measure TOB-2a. Stop processing.
b. If Tobacco Use Status equals 1, continue processing and proceed to recheck Tobacco Use Treatment FDA-Approved Cessation Medication.
18. Recheck Tobacco Use Treatment FDA-Approved Cessation Medication
a. If Tobacco Use Treatment FDA-Approved Cessation Medication equals 2, the case will proceed to Measure Category Assignment of D and will be in the Measure Population for sub-measure TOB-2a. Stop processing.
b. If Tobacco Use Treatment FDA-Approved Cessation Medication equals 1, the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population for sub-measure TOB-2a. Stop processing.
c. If Tobacco Use Treatment FDA-Approved Cessation Medication equals 3, continue processing and proceed to recheck Reason for No Tobacco Cessation Medication During Hospital Stay.
19. Recheck Reason for No Tobacco Cessation Medication During Hospital Stay
a. If Reason for No Tobacco Cessation Medication During Hospital Stay equals N, the case will proceed to Measure Category Assignment of D and will be in the Measure Population for sub-measure TOB-2a. Stop processing.
b. If Reason for No Tobacco Cessation Medication During the Hospital Stay equals Y, the case will proceed to a Measure Category Assignment of E and will be in the Numerator Population for sub-measure TOB-2a. Stop processing.
CPT® only copyright 2023 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.