Measure Information Form
Version 2023B
Measure Information Form
The measure is reported as an overall rate which includes all newborns that were exclusively fed breast milk during the entire hospitalization.
Rationale: Exclusive breast milk feeding for the first 6 months of neonatal life has long been the expressed goal of World Health Organization (WHO), Department of Health and Human Services (DHHS), American Academy of Pediatrics (AAP) and American College of Obstetricians and Gynecologists (ACOG). ACOG has recently reiterated its position (ACOG, 2018). A Cochrane review substantiates the benefits (Kramer et al., 2012). Much evidence has now focused on the prenatal and intrapartum period as critical for the success of exclusive (or any) breastfeeding (Centers for Disease Control and Prevention [CDC], 2020; CDC, 2013; Petrova et al., 2007; Taveras et al., 2004). Exclusive breast milk feeding rate during birth hospital stay has been calculated by the California Department of Public Health for the last several years using newborn genetic disease testing data. Healthy People 2020 and the CDC have also been active in promoting this goal. Type Of Measure: Process Improvement Noted As: Increase in the rateIncluded Populations: Not applicable Excluded Populations: None Data Elements:Denominator Statement: Single term newborns discharged alive from the hospital
Included Populations: Liveborn newborns with ICD-10-CM Principal Diagnosis Code for single liveborn newborn as defined in Appendix A, Table 11.20.1 Single Liveborn Newborn Excluded Populations:Data Elements:
- Admitted to the Neonatal Intensive Care Unit (NICU) at this hospital during the hospitalization
- ICD-10-CM Other Diagnosis Codes for galactosemia as defined in Appendix A, Table 11.21 Galactosemia
- ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for parenteral nutrition as defined in Appendix A, Table 11.22 Parenteral Nutrition
- Experienced death
- Length of Stay >120 days
- Patients transferred to another hospital
- Patients who are not term or with < 37 weeks gestation completed
- American Academy of Pediatrics. Section on Breastfeeding. Policy Statement. Breastfeeding and the Use of Human Milk. Pediatrics 2012 Mar; 129 (3): e827-841.
- ACOG Committee Opinion No. 756: Optimizing Support for Breastfeeding as Part of Obstetric Practice. (2018). Obstetrics and gynecology, 132(4), e187–e196. https://doi.org/10.1097/AOG.0000000000002890
- California Department of Public Health. (2017). Division of Maternal, Child and Adolescent Health, Breastfeeding Initiative, In-Hospital Breastfeeding Initiation Data, Hospital of Occurrence: Available at: https://www.cdph.ca.gov/Programs/CFH/DMCAH/Breastfeeding/Pages/In-Hospital-Breastfeeding-Initiation-Data.aspx
- Centers for Disease Control and Prevention. (2020). Division of Nutrition, Physical Activity and Obesity. Breastfeeding Report Card. Available at: https://www.cdc.gov/breastfeeding/data/reportcard.htm
- Centers for Disease Control and Prevention. Strategies to Prevent Obesity and Other Chronic Diseases: The CDC Guide to Strategies to Support Breastfeeding Mothers and Babies. Atlanta: U.S. Department of Health and Human Services; 2013. Available at: https://www.cdc.gov/breastfeeding/pdf/bf-guide-508.pdf
- Feltner, C., Weber, R. P., Stuebe, A., Grodensky, C. A., Orr, C., & Viswanathan, M. (2018). Breastfeeding Programs and Policies, Breastfeeding Uptake, and Maternal Health Outcomes in Developed Countries. Agency for Healthcare Research and Quality (US).
- Kramer, M. S., & Kakuma, R. (2012). Optimal duration of exclusive breastfeeding. The Cochrane database of systematic reviews, 2012(8), CD003517. https://doi.org/10.1002/14651858.CD003517.pub2
- Perrine CG, Chiang KV, Anstey EH, et al. Implementation of Hospital Practices Supportive of Breastfeeding in the Context of COVID-19 — United States, July 15–August 20, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1767–1770. DOI: http://dx.doi.org/10.15585/mmwr.mm6947a3
- Petrova, A., Hegyi, T., & Mehta, R. (2007). Maternal race/ethnicity and one-month exclusive breastfeeding in association with the in-hospital feeding modality. Breastfeeding Medicine. 2(2):92-8.
- Taveras, E.M., Li, R., Grummer-Strawn, L., Richardson, M., Marshall, R., Rego, V.H., Miroshnik, I., & Lieu, T.A. (2004). Opinions and practices of clinicians associated with continuation of exclusive breastfeeding. Pediatrics. 113(4):e283-90.
- US Department of Health and Human Services. (2020). Healthy People 2020 Final Review. Washington, DC: US Department of Health and Human Services. Available at: https://www.cdc.gov/nchs/healthy_people/hp2020-final-review.htm
- World Health Organization. (2007). Indicators for assessing infant and young child feeding practices. Washington, DC, USA: World Health Organization. Available at: http://apps.who.int/iris/bitstream/10665/43895/1/9789241596664_eng.pdf
California Maternal Quality Care Collaborative
PC-05: Exclusive Breast Milk Feeding Algorithm Narrative
Numerator: Newborns that were fed breast milk only since birthDenominator: Single term newborns discharged alive from the hospital
Variable Key: Length of Stay
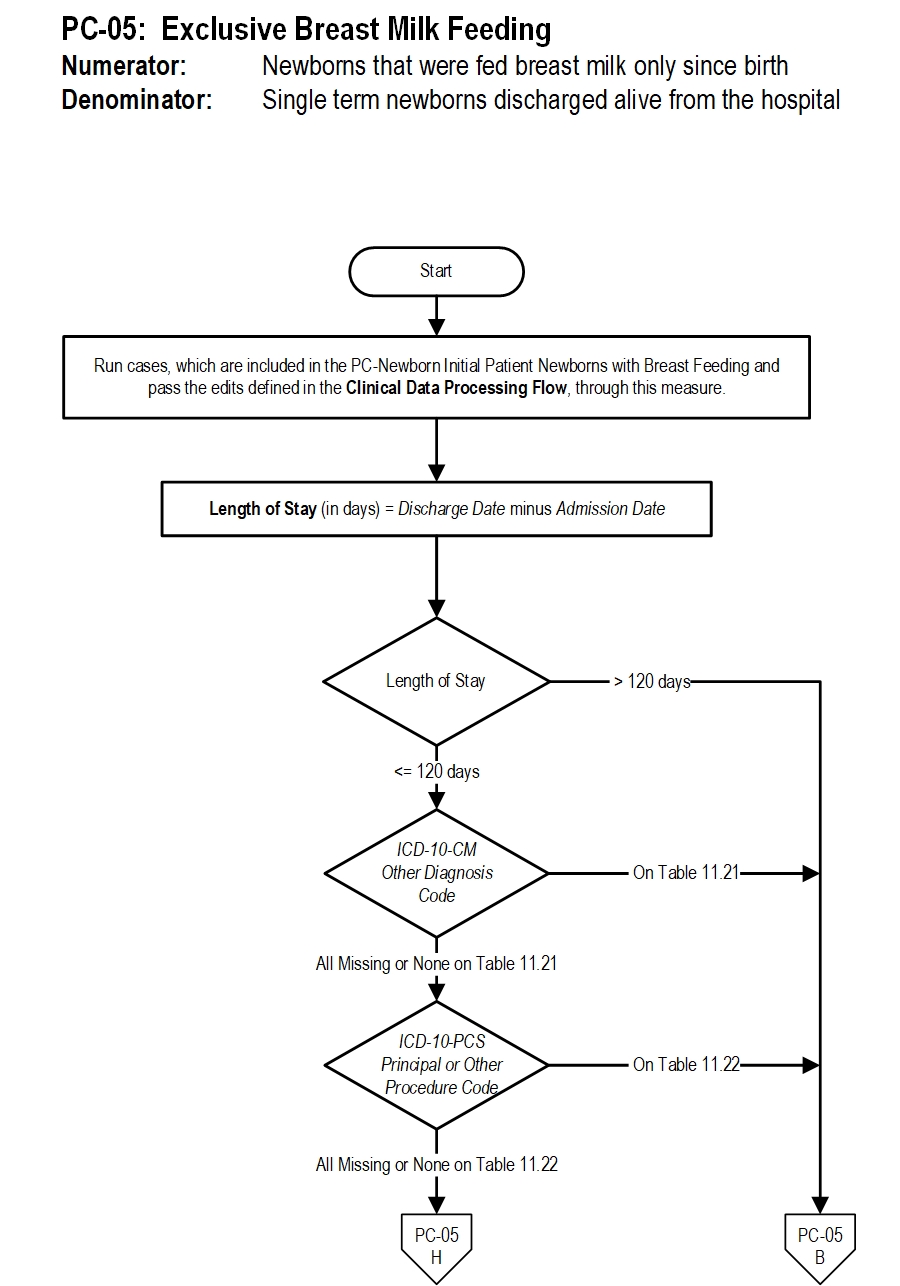
1. Start processing. Run cases, which are included in the PC Newborn Initial Patient Newborns with Breast Feeding and pass the edits defined in the Clinical Data Processing Flow , through this measure.
2. Calculate Length of Stay. Length of Stay, in days, is equal to the Discharge Date minus the Admission Date. continue processing and proceed to check Length of Stay.
3. Check Length of Stay
- If Length of Stay greater than 120 days, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Length of Stay equals to or less than 120 days, continue processing and proceed to check ICD-10-CM Other Diagnosis Codes.
- If at least one of the ICD-10-CM Other Diagnosis Codes is on Table 11.21, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If all ICD-10-CM Other Diagnosis Codes are missing or none of them on Table 11.21, continue processing and proceed to check ICD-10-PCS Principal or Other Procedure Codes.
- If at least one of the ICD-10-PCS Principal or Other Procedure Codes is on Table 11.22, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If all ICD-10-PCS Principal or Other Procedure Codes are missing or none of them on Table 11.22, continue processing and proceed to check Discharge Disposition.
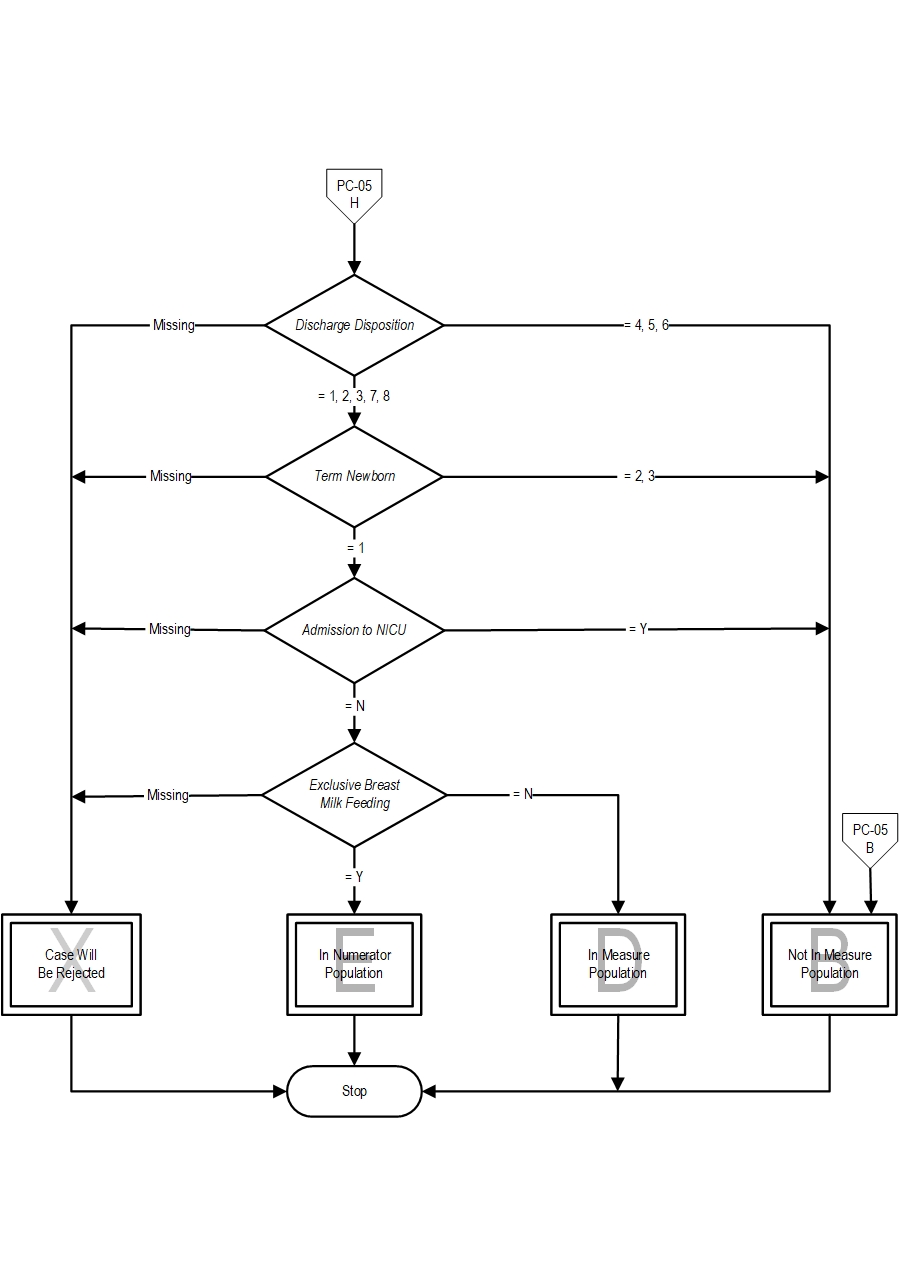
- If Discharge Disposition is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Discharge Disposition equals 4, 5 or 6, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Discharge Disposition equals 1, 2, 3, 7 or 8, continue processing and proceed to check Term Newborn.
- If Term Newborn is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Term Newborn equals 2 or 3, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
- If Term Newborn equals 1, continue processing and proceed to check Admission to NICU.
- If Admission to NICU is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Admission to NICU equals Y, the case will proceed to a Measure Category Assignment of B and will not be in the Measure Population. Stop processing.
ca. If Admission to NICU equals N, continue processing and proceed to check Exclusive Breast Milk Feeding.
- If Exclusive Breast Milk Feeding is missing, the case will proceed to a Measure Category Assignment of X and will be rejected. Stop processing.
- If Exclusive Breast Milk Feeding equals Y, the case will proceed to a Measure Category Assignment of E and will be in the numerator population. Stop processing.
- If Exclusive Breast Milk Feeding equals N, the case will proceed to a Measure Category Assignment of D and will be in the population. Stop processing.
CPT® only copyright 2023 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.