Measure Information Form
Version 2023A1
Measure Information Form
Influenza (flu) is an acute, contagious, viral infection of the nose, throat and lungs (respiratory illness) caused by influenza viruses. Outbreaks of seasonal influenza occur annually during late autumn and winter months although the timing and severity of outbreaks can vary substantially from year to year and community to community. Influenza activity most often peaks in February, but can peak rarely as early as November and as late as April. In order to protect as many people as possible before influenza activity increases, most flu vaccine is administered in September through November, but vaccine is recommended to be administered throughout the influenza season as well. Because the flu vaccine usually first becomes available in September, health systems can usually meet public and patient needs for vaccination in advance of widespread influenza circulation.
Rationale: Up to 1 in 5 people in the United States get influenza every season (CDC, Key Facts 2015). Each year an average of approximately 226,000 people in the US are hospitalized with complications from influenza and between 3,000 and 49,000 die from the disease and its complications (Thompson 2003). Combined with pneumonia, influenza is the nation’s 8th leading cause of death (Heron 2012). Up to two-thirds of all deaths attributable to pneumonia and influenza occur in the population of patients that have been hospitalized during flu season regardless of age (Fedson 2000). The Advisory Committee on Immunization Practices (ACIP) recommends seasonal influenza vaccination for all persons 6 months of age and older to highlight the importance of preventing influenza. Vaccination is associated with reductions in influenza among all age groups (Kostova 2013).The influenza vaccination is the most effective method for preventing influenza virus infection and its potentially severe complications. Screening and vaccination of inpatients is recommended, but hospitalization is an underutilized opportunity to provide vaccination to persons 6 months of age or older.
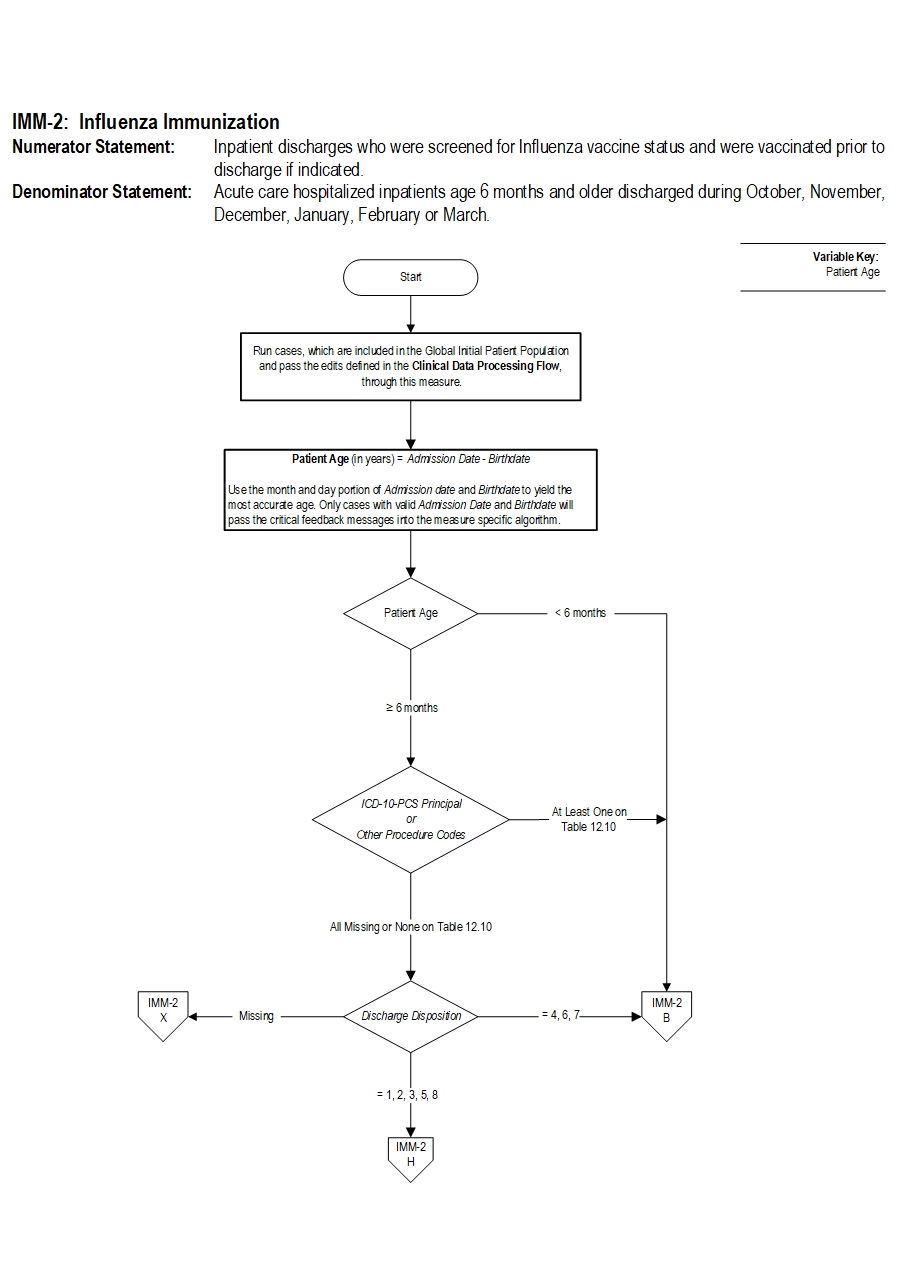
Type Of Measure: Process Improvement Noted As: Increase in the rateIncluded Populations:Denominator Statement: Acute care hospitalized inpatients age 6 months and older discharged during October, November, December, January, February or March.Excluded Populations: None Data Elements:
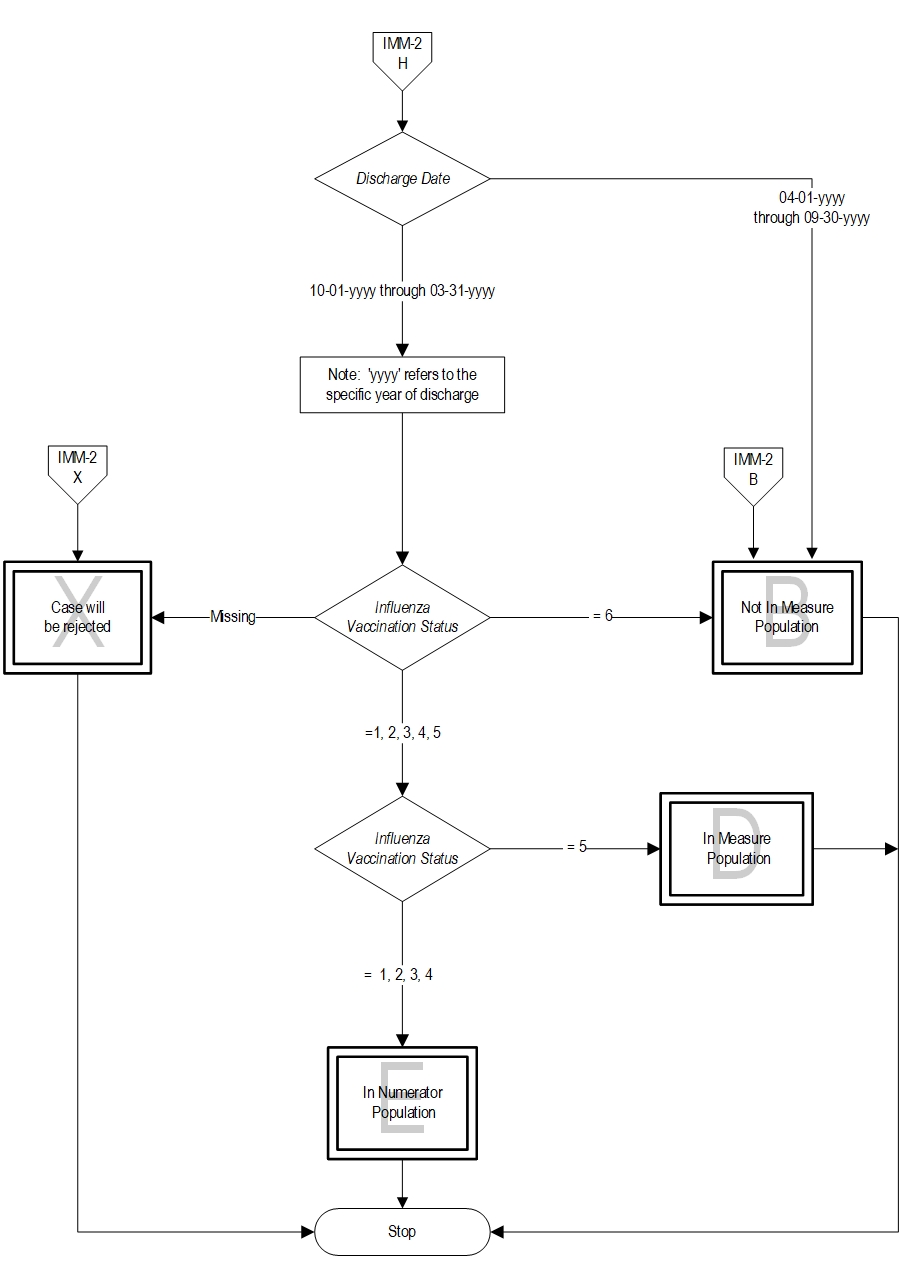
- Patients who received the influenza vaccine during this inpatient hospitalization
- Patients who received the influenza vaccine during the current year’s flu season but prior to the current hospitalization
- Patients who were offered and declined the influenza vaccine
- Patients who have an allergy/sensitivity to the influenza vaccine, anaphylactic latex allergy or anaphylactic allergy to eggs, or for whom the vaccine is not likely to be effective because of bone marrow transplant within the past 6 months, or history of Guillian-Barre syndrome within 6 weeks after a previous influenza vaccination, or symptomatic suspected or confirmed COVID-19
Included Populations: Inpatient discharges 6 months of age and older Excluded Populations:Data Elements:
- Patients less than 6 months of age
- Patients who expire prior to hospital discharge
- Patients with an organ transplant during the current hospitalization(Appendix A, Table 12.10)
- Patients for whom vaccination was indicated, but supply had not been received by the hospital due to problems with vaccine production or distribution
- Patients who have a Length of Stay greater than 120 days
- Patients who are transferred or discharged to another acute care hospital
- Patients who leave Against Medical Advice (AMA)
- Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants.CID. December 2010; 51 (12): 1355-1361.
- Carr S, Allison Kim J, et al. Safety and immunogenicity of live attenuated and inactivated influenza vaccines in children with cancer. J Infect Dis.2011:204:1475-1482.
- Centers for Disease Control and Prevention. (2013). Estimated Applications/LocalApps.Influenza Illnesses and Hospitalizations Averted by Influenza Vaccination — United States,2012–13 Influenza Season. MMWR. 2013;62(49):997-1000.
- Centers for Disease Control and Prevention. (2015). Key facts about influenza and the influenza vaccine, October 2015. Available at:http://www.cdc.gov/flu/keyfacts.htm. Accessed October 14, 2015
- Centers for Disease Control and Prevention. (2013). Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2013-2014. MMWR, September 20,2013; 62(RR07); 1-43. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6207a1.htm
- Centers for Disease Control and Prevention. (2015). Prevention and Control of Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2015–16 Influenza Season. MMWR,August 7, 2015; 64(30);818-825. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6430a3.htm
- Centers for Disease Control and Prevention. (2017). Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2017-2018. MMWR, August 17, 2017;62(RR07); 1-20. https://www.cdc.gov/mmwr/volumes/66/rr/rr6602a1.htm
- Chung EY, Huang L, and Schneider L. Safety of influenza vaccine administration in egg-allergic patients. Pediatrics. 2010 May;125(5):e1024-30. Epub 2010 Apr 5.
- Darvishiana M, Gefenaitea G, Turnerc RM, Pechlivanogloua P, Van der HoekeW, Van den Heuvelb ER, Haka E (2014). After adjusting for bias in meta-analysis seasonal influenza vaccine remains effective in community-dwelling elderly. JClin Epidemiol 2014;67:734-744.
- Fedson DS, Houck PM, Bratzler DW. Hospital-based influenza and pneumococcal vaccination: Sutton’s Law applied to prevention. Infect ControlHosp Epi. 2000;21:692-699.
- Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 Influenza Season. MMWR Recomm Rep 2021;70(No. RR-5):1–28. DOI: http://dx.doi.org/10.15585/mmwr.rr7005a1
- Heron M (2015). Deaths: Leading Causes for 2012. National Vital StatisticsReports; vol 64 no 10. Hyattsville, MD: National Center for Health Statistics.2015.
- Kostova D, Reed C, Finelli L, Cheng P, Gargiullo PM, Shay DK, Singleton JA,Meltzer MI, Lu P, Joseph S. (2013). Influenza Illness and HospitalizationsAverted by Influenza Vaccination in the United States, 2005–2011. PLoS One.2013; 8(6): e66312.
- Mandell LA, Wunderink RG, Anzueta A, Bartlett JG, Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007 March 1;44 Suppl2:S27-72.
- Nichol KL, Nordin J, Mullooly J, et al. Influenza Vaccination and Reduction in Hospitalizations for Cardiac Disease and Stroke among the Elderly. N Engl JMed. 2003;348:1322-1332.
- Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, et al.(2015). Estimating Influenza Disease Burden from Population-Based Surveillance Data in the United States. PLoS ONE 10(3): e0118369
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ,Fukuda. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003 January 8; 289 (2): 179-186.
CPT® only copyright 2022 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.