Measure Information Form
Version 2023A1
Measure Information Form
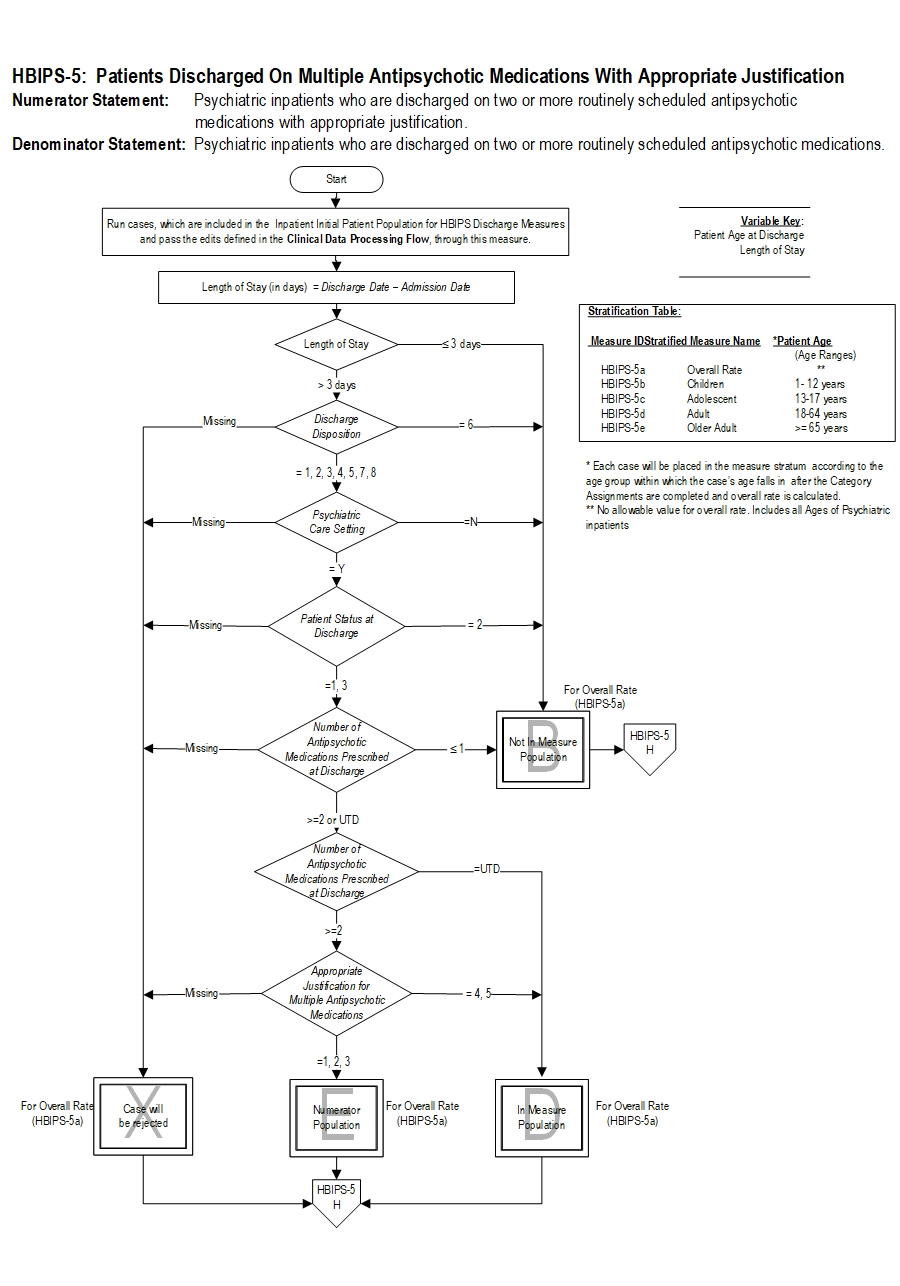
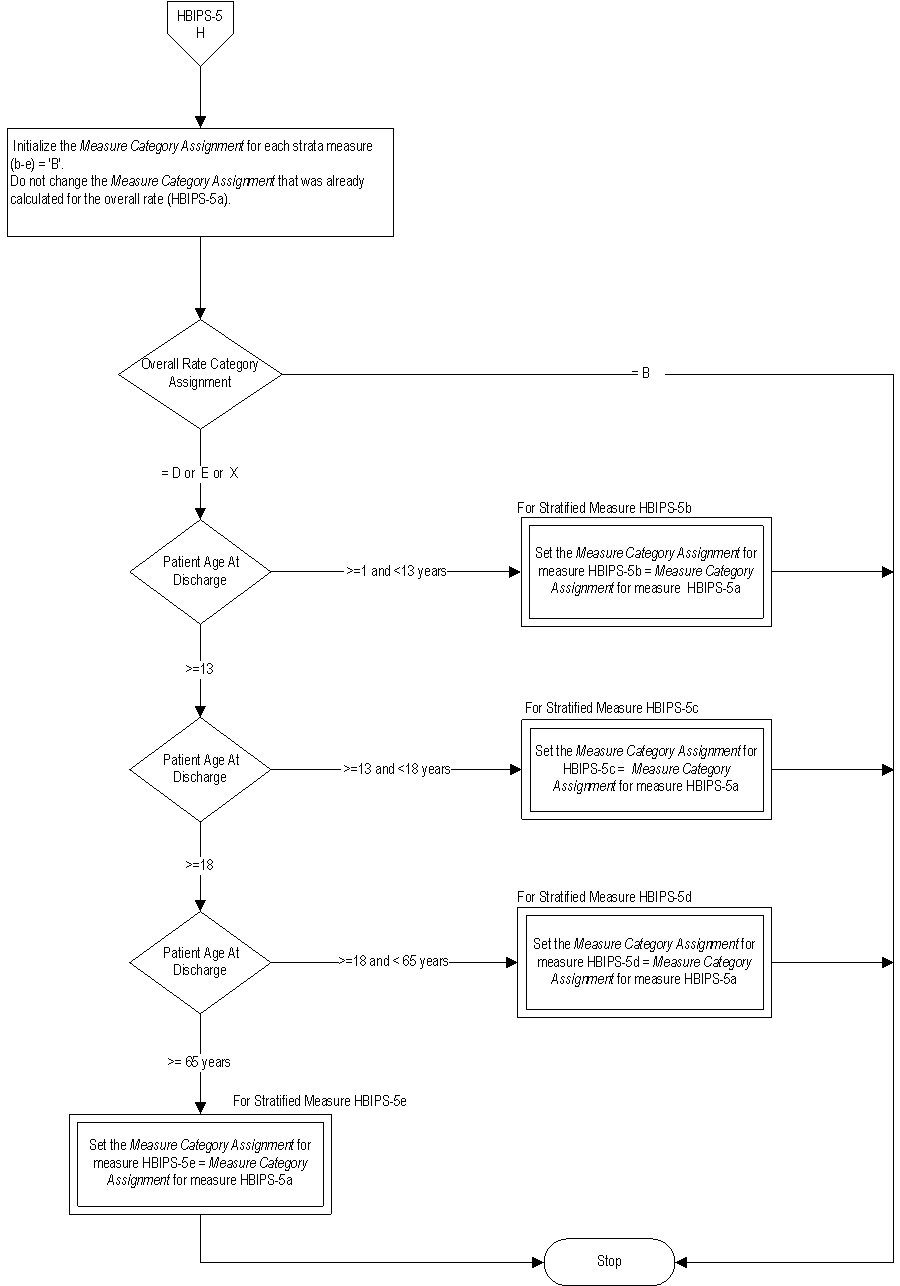
Included Populations: Not applicable Excluded Populations: None Data Elements:Denominator Statement: Psychiatric inpatient discharges
Included Populations:Excluded Populations:
- Patients with ICD-10-CM Principal or Other Diagnosis Codes for Mental Disorders as defined in Appendix A, Table 10.01 discharged on two or more routinely scheduled antipsychotic medications (refer to Appendix C, Table 10.0- Antipsychotic Medications).
Data Elements:
- Patients who expired
- Patients with an unplanned departure resulting in discharge due to elopement
- Patients with an unplanned departure resulting in discharge due to failing to return from leave
- Patients with a length of stay ≤ 3 days
- American Psychiatric Association (APA). (2004). Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 161(2 Suppl):1-56
- Ananth, J., Parameswaran, S., & Gunatilake, S. (2004). Antipsychotic polypharmacy comparing monotherapy with polypharmacy and augmentation. Curr Med Chem. 11(3):313-327 Curr Pharm Des. 10(18):2231-2238.
- Centorrino, F., Gören, J.L., Hennen, J., Salvatore, P., Kelleher, J.P., & Baldessarini, R.J. (2004) Multiple versus single antipsychotic agents for hospitalized psychiatric patients: a case control study of risk versus benefit. Am J Psychiatry. 161 (4):700-706.
- Covell, N.H., Jackson, C.T., Evans, A.C., & Essock, S.M. (2002). Antipsychotic prescribing practices in Connecticut's public mental health system: rates of changing medication prescribing styles. Schiz Bull. 28(1):17-29,
- Ganguly, R., Kotzan, J.A., Miller, L.S., Kennedy, K., & Martin, B.C. (2004). Prevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998-2000. J Clin Psychiatry. 65(10):1377-88.
- Gilmer, T.P., Dolder, C.R., Folsom, D.P., Mastin, W., & Jeste, D.V. (2007), Antipsychotic polypharmacy trends among Medicaid beneficiaries with schizophrenia in San Diego County, 1999 - 2004. Psychiatric Serv. 59(7):1007-1010.
- Jaffe, A.B. & Levine, J. (2003). Antipsychotic medication co-prescribing in a large state hospital system. Pharmacoepidemiol Drug Saf.12:41-48.
- Kreyenbuhl, J., Valenstein, M., McCarthy, J.F., Ganocyz, D., & Blow, F.C. (2006). Long-term combination antipsychotic treatment in VA patients with schizophrenia. Schiz Res.84:90-99.
- National Association of State Mental Health Program Directors (NASMHPD). (2001).Technical report on psychiatric polypharmacy. Alexandria, VA.
- Potkin, S.G., Thyrum, P.T., Alva, G., Bera, R., Yeh, C., & Arvanitis, L.A. (2002). The safety and pharmacokinetics of quetiapine when coadministered with haloperidol, risperidone or thioridazine. J Clin Psychopharmacol. 22:121-130.
- Shim, J.C., Shin, J.G., Kelly, D.L., Jung, D.U., Seo, Y.S., Liu, K.H., et al. (2007). Adjunctive treatment with a dopamine partial agonist aripiprazole, for treatment of antipsychotic-induced hyperprolactinemia: A placebo controlled trial. Am J Psych.164:1404-1410.
- Stahl, S.M. & Grady, M.M. (2004). A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy augmentation. Curr Med Chem.11:313-327.
- Tranulis, C., Skalli, L., Lalonde, P., & Nicole, L. (2008). Benefits and risks of antipsychotic polypharmacy. An evidence based review of the literature. Drug Saf.31(1):7-20
- University HealthSystem Consortium. (2006). Mental health performance measures field brief. Oakbrook, IL.
CPT® only copyright 2022 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.