Measure Information Form
Version 2023A
Measure Information Form
**SUSPENDED Effective January 1, 2016**Since “time is brain”, the overall speed of the revascularization process is an important and appropriate measure. In multicenter clinical trials of catheter-directed therapies, the probability of good outcome as defined by a Modified Rankin Score of 0-2 at 90 days decreased as time to angiographic revascularization increased. It is estimated that for every 30-minute delay in time to revascularization, there is a 10% decrease in the likelihood of a good outcome from endovascular reperfusion therapy.
Type Of Measure: Process Improvement Noted As: Decrease in the median valueIncluded Populations:Excluded Populations:
- Discharges with ICD-10-CM Principal Diagnosis Code for ischemic stroke as defined in Appendix A, Table 8.1 for ICD-10 codes,
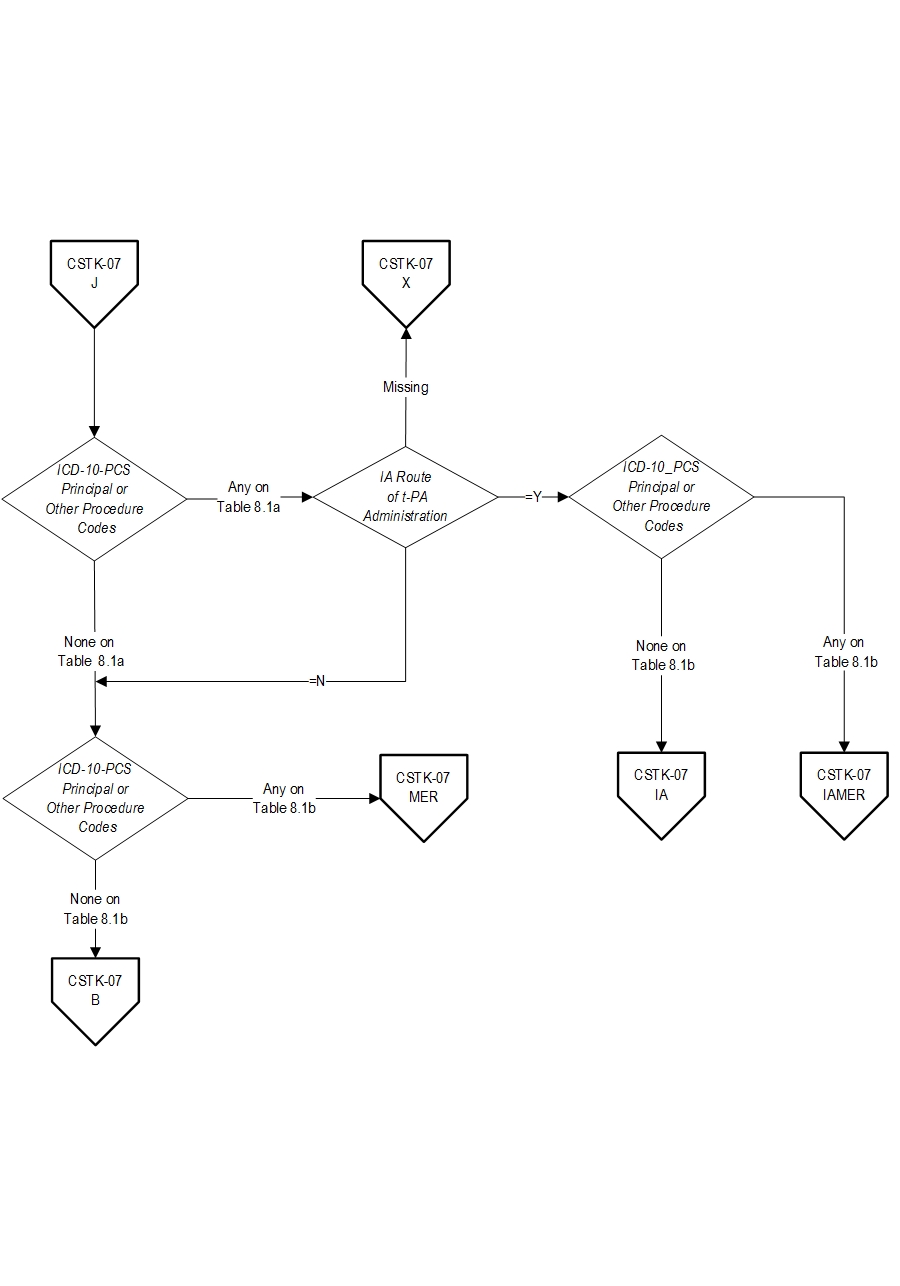
AND- Patients with documented Thrombolytic Infusion Therapy (ICD-10-PCS Principal or Other Procedure Codes as defined in Appendix A, Table 8.1a for ICD-10 codes) OR Mechanical Endovascular Reperfusion Therapy (ICD-10-PCS Principal or Other Procedure Codes as defined in Appendix A, Table 8.1b for ICD-10 codes).
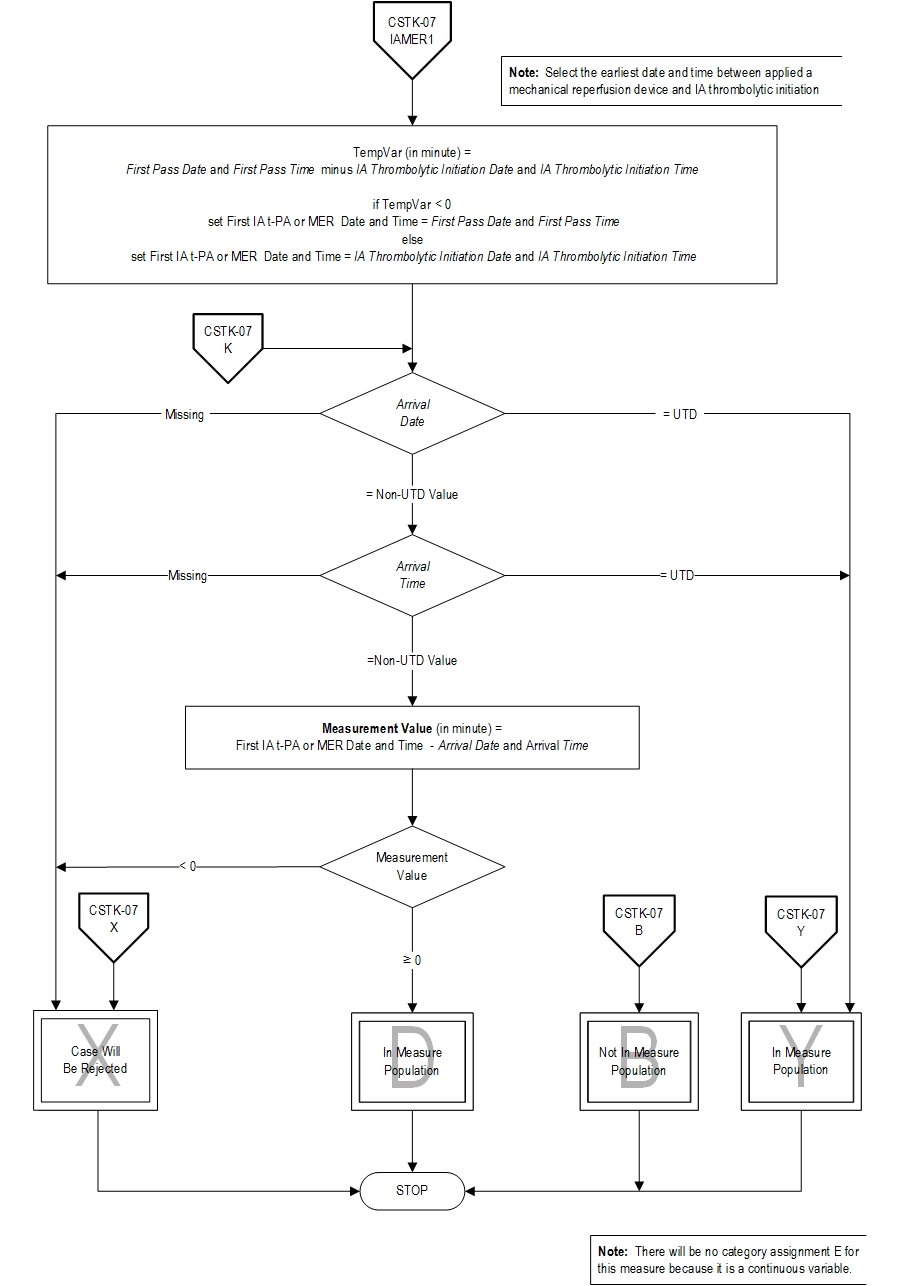
Data Elements:
- Patients less than 18 years of age
- Patients who have a Length of Stay > 120 days
- Patients admitted for Elective Carotid Intervention
- Admission Date
- Arrival Date
- Arrival Time
- Birthdate
- Discharge Date
- Elective Carotid Intervention
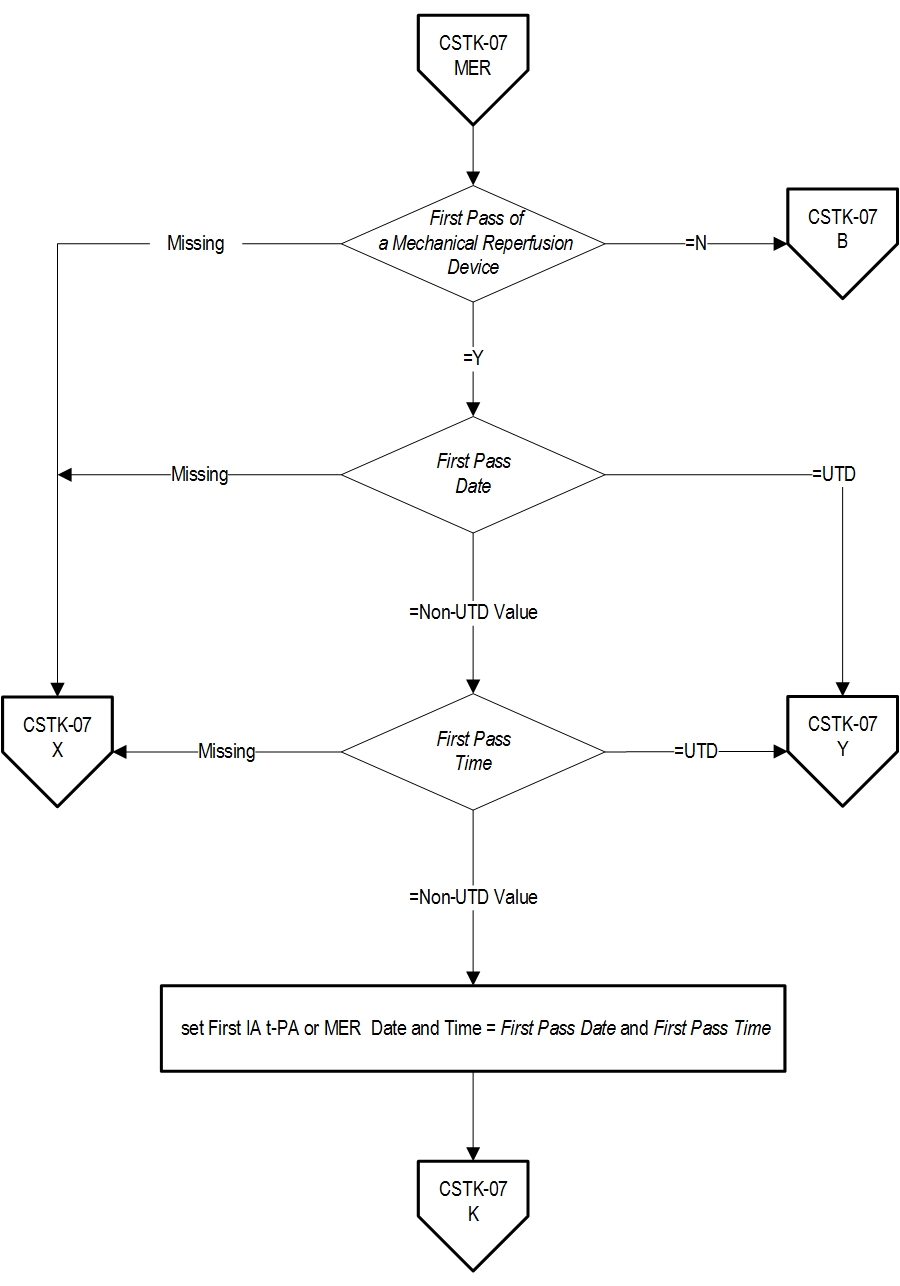
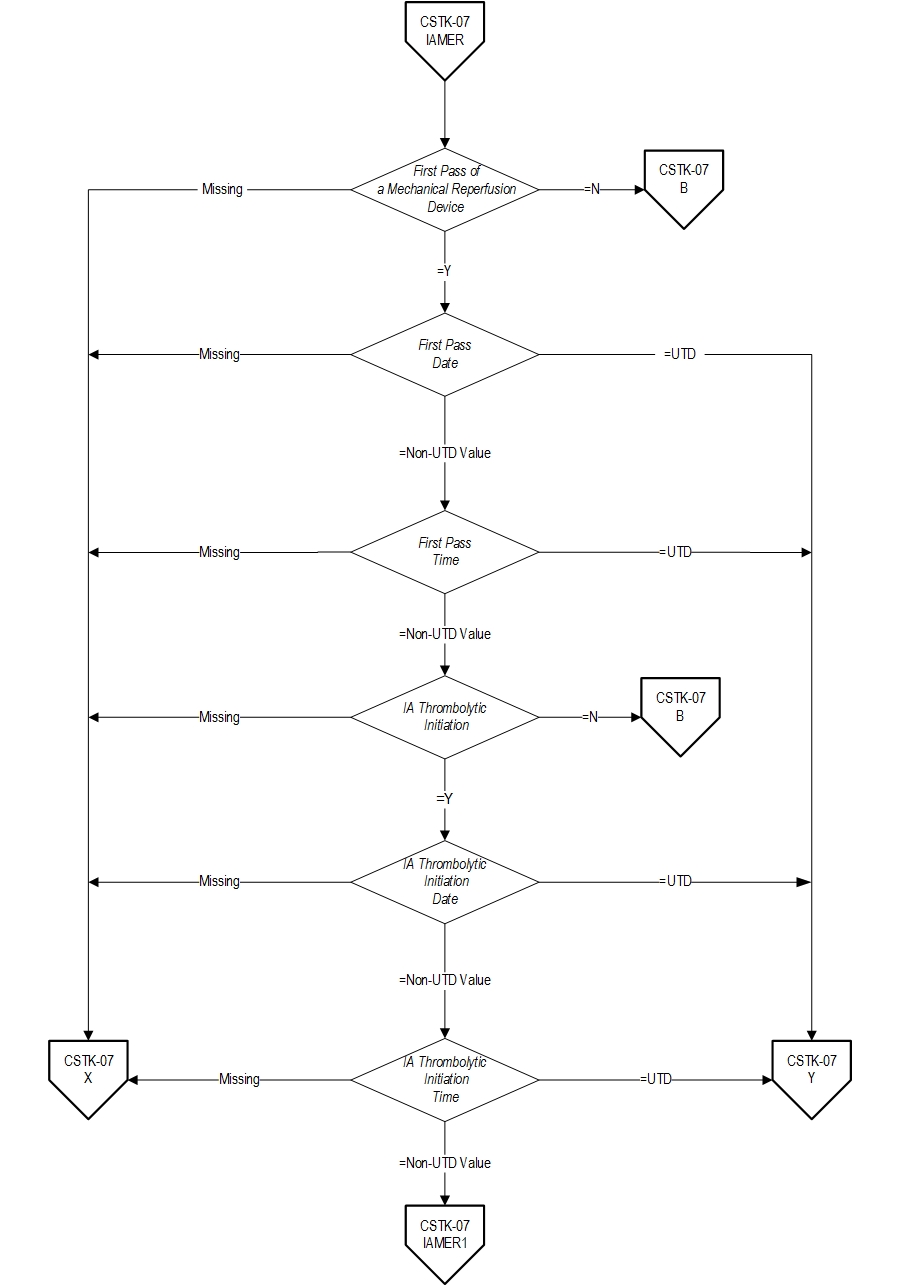
- First Pass Date
- First Pass Time
- First Pass of a Mechanical Reperfusion Device
- IA Route of Alteplase Administration
- IA Thrombolytic Initiation
- IA Thrombolytic Initiation Date
- IA Thrombolytic Initiation Time
- ICD-10-CM Principal Diagnosis Code
- ICD-10-PCS Other Procedure Codes
- ICD-10-PCS Other Procedure Dates
- ICD-10-PCS Principal Procedure Code
- ICD-10-PCS Principal Procedure Date
2. Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). 2004.
3. Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. 2007 focused update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction). J Am Coll Cardiol. 2008;51:210—-47. 4. Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et. al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. NEJM. 2015 Mar;372(11): 1009-17. 5. Demchuk AM, Goyal M, Monon BK, Eesa M, Ryckborst KJ, Kamal N, et. al. Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times (ESCAPE) trial: methodology. Int J Stroke. 2015 Apr;10(3): 429-38. 6. Furlan A, Higashida R, Wechsler L, et. al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study; a randomized controlled trial. Prolyse in Actue Cerebral Thromboembolism. JAMA. 1999;282:2003-2011. 7. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:32-36. 8. Khatari P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA; IMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009 Sep 29;73(13):1066-72. 9. Khatari P, Hill MD, Palesch YY, et. al. Methodology of the Interventional Manaagement of Stroke III Trial. Int J Stroke. 2008;3:130-137. 10. Leifer D, Bravata DM, Connors JJ III, Hinchey JA, Jauch EC, Johnston SC, Latchaw R, Likosky W, Ogilvy C, Qureshi AI, Summers D, Sung GY, Williams LS, Zorowitz R, on behalf of the American Heart Association Special Writing Group of the Stroke Council, Atherosclerotic Peripheral Vascular Disease Working Group and Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular Nursing. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow-up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42; 857. 11. Penumbra Pivotal Stroke Trial Investigators. The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761-2768. 12. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 13. Sacks D, Black CM, Cognard C, Connors JJ III, Frei D, Gupta R, Jovin TG, Kluck B, Meyers PM, Murphy KJ, Ramee S, Rϋfenacht DA, Stallmeyer MJB, Vorwerk D. Multisociety consensus quality improvement guidelines for intraarterial catheter-directed treatment of acute ischemic stroke from the American Society of Neuroradiology, Canadian Interventional Radiology Association, Cardiovascular and interventional Radiological Society of Europe, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, European Society of Minimally Invasive Neurological Therapy, and Society of Vascular and Interventional Neurology. J Vasc Interv Radiol. 2013;24:151-163. 14. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et. al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. NEJM. 2015 Apr: 1-11. 15. Sharma VK, Teoh HL, Wong LYH, Su J, Ong BKC, and Chan BLP. Recanalization therapies in acute ischemic stroke: pharmacological agents, devices, and combinations. Stroke Research and Treatment. 2010. 16. Smith WS, Sung G, Saver J, et. al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205-1212.17. Tarr R, Hsu D, Kulcsar Z, et. al. The POST trial: initial post-market experience of the Penumbra system: revascularization of large vessel occlusion in acute ischemic stroke in the United States and Europe. _J Neurointerv Surg. 2010;2:341-344.
18. Turk AS, Frei D, Fiorella D, Mocco J, Baxter B, Siddiqui A, et. al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg. 2014 May;694): 260-4.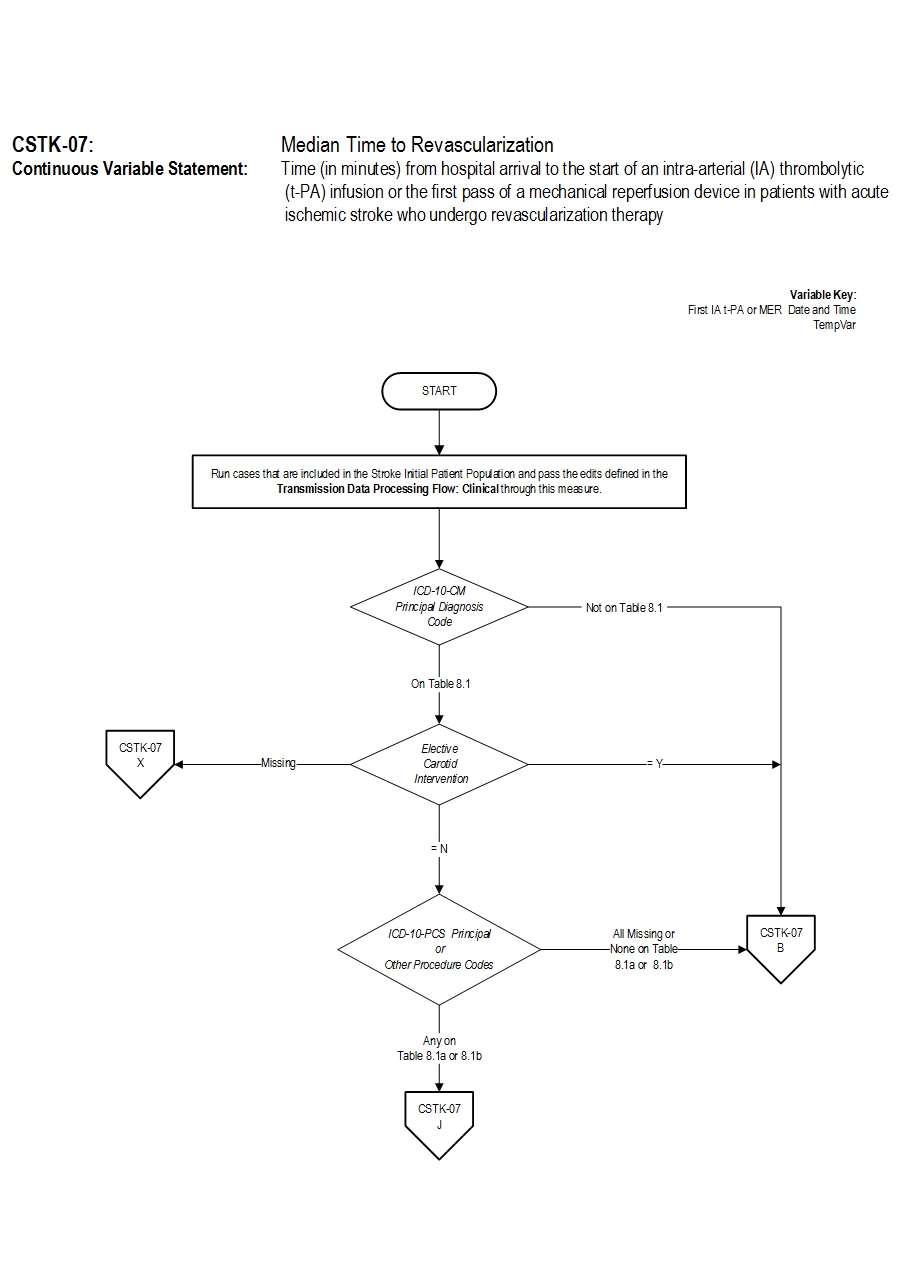

CPT® only copyright 2022 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.