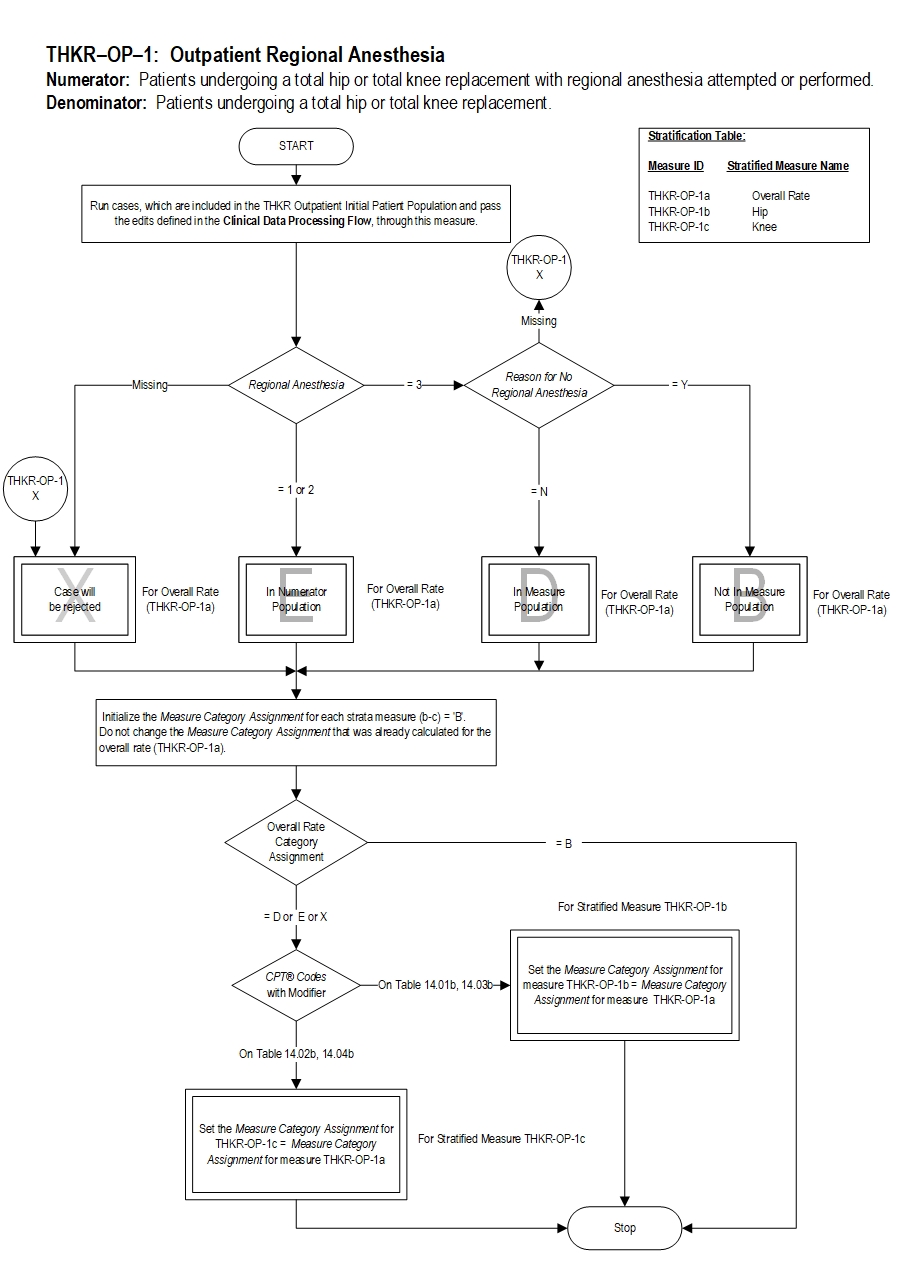
Measure Information Form
Version 2022B2
Measure Information Form
| Set Measure ID | Performance Measure Name |
|---|---|
| THKR-OP-1a |
Regional Anesthesia - Hip and Knee Overall |
| THKR-OP-1b |
Regional Anesthesia - Hip |
| THKR-OP-1c |
Regional Anesthesia - Knee |
Included Populations: Patients receiving any of the following or documentation of a failed attempt of any of the following during the operative episode:Denominator Statement: Patients undergoing a total hip or total knee replacement.Excluded Populations: None Data Elements:
- Epidural anesthesia
- Epidural block
- Peripheral nerve block (single injection or continuous infusion)
- Spinal anesthesia
- Spinal block
- Subarachnoid block
Included Populations:Excluded Populations:
- Patients with a CPT® Code with Modifier as defined in Appendix A: Table 14.01b (Total Hip Replacements-OP), or Table 14.02b (Total Knee Replacements-OP), or Table 14.03b (Bilateral Hip Replacements-OP) or Table 14.04b (Bilateral Knee Replacements-OP)
Data Elements:
- Patients less than 18 years of age
- Patients with a CPT® Code with Modifier as defined in Appendix A: Table 14.05b (partial hip and partial knee replacements-OP), or Table 14.06b (revision and resurfacing procedures-OP), or Table 14.07b (removal of implanted devices/prostheses-OP)
- Patients with an ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes as defined in Appendix A: Table 14.08 (complication of internal fixation device/prosthesis, or Table 14.09 (malignant neoplasm of the pelvis, sacrum, coccyx, lower limbs, or bone/bone marrow or a disseminated malignant neoplasm)
- Documented contraindication by physician/APN/PA (e.g. anticoagulated patients, coagulopathies, neurologic condition, previous spinal fusion) clearly indicated as reason for no regional anesthesia
- 1 Memtsoudis SG, Xuming S.; Ya-Lin Chiu, et al. Perioperative Comparative Effectiveness of Anesthetic Technique in Orthopedic Patients, Anesthesiology 05 2013, Vol.118, 1046-1058.
- 2 Mauermann WJ, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a metaanalysis. Anesth. Analg. 2006; 103: 1018–25.
- 3 Hu S, Zhang Z-Y, Hua Y-Q, Li J, Cai Z-D. A comparison of regional and general anaesthesia for total replacement of the hip or knee: a metaanalysis. J. Bone Joint Surg. Br. 2009; 91: 935–42.
- 4 Zorrilla-Vaca A, Grant MC, Mathur V, Li J, Wu CL. The Impact of Neuraxial Versus General Anesthesia on the Incidence of Postoperative Surgical Site Infections Following Knee or Hip Arthroplasty: A Meta-Analysis. Regional Anesthesia & Pain Medicine: September/October 2016 - Volume 41 - Issue 5 - p 555–563.
- 5 Kim JH, Cho MR, et al. A comparison of femoral/sciatic nerve block with lateral femoral cutaneous nerve block and combined spinal epidural anesthesia for total knee replacement arthroplasty. Korean J Anesthesiol 2012 May 62(5): 448-453.
- 6 Liu JL, Yuan WX, et al. Peripheral nerve blocks versus general anesthesia for total knee replacement in elderly patients on the postoperative quality of recovery. Clinical Interventions in Aging 2014:9 341-350.
- 7 Surgical Management of Osteoarthritis of the Knee Evidence-Based Clinical Practice Guideline. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors, 12/4/2015.
- 8 Management of Osteoarthritis of the Hip Evidence-Based Clinical Practice Guideline. Adopted by the American Academy of Orthopaedic Surgeons Board of Directors, 3.13.17.
- 9 Memtsoudis SG, Thomas Danninger, Rehana Rasul, Jashvant Poeran, Philipp Gerner, Ottokar Stundner, Edward R. Mariano, Madhu Mazumdar. Inpatient Falls after Total Knee Arthroplasty. Anesthesiology, 2014; 120 (3): 551-563.
- Nielsen PT, Jørgensen LN, Albrecht-Beste E, LeffersA, RasmussenLS. Lower thrombosis risk with epidural blockade in knee arthroplasty. Acta Orthopaedica Scandinavica, 1990,61:1, 29-31
- Mitchell D, Friedman, RJ, Baker DJ, Cooke JE, Darcy, MD, Miller MC. Prevention of thromboembolic disease following total knee arthroplasty: Epidural versus general anesthesia. Clinical Orthopaedics & Related Research, August 1991; 269:109-112.
- Jorgensen LN, Rasmussen LS, Nielsen PT, Leffers A, Albrecht-Beste E. Antithrombotic efficacy of continuous extradural analgesia after knee replacement. Br J Anaesth. 1991/1; 1: 8-12
- Soohoo NF, Lieberman JR, et al. Development of Quality of Care Indicators for Patients Undergoing THR/TKR. BMJ Qual Saf 2011;20:153-157
- Basques BA, Toy JO, Bohl, DD, Golinvaux, NS, Grauer, JN. General Compared with Spinal Anesthesia for Total Hip Arthroplasty. The Journal of Bone and Joint Surgery 2015;97:455-61
- Hunt LP, Ben-Shlomo Y, Clark EM, Dieppe P, Judge A, MacGregor AJ, Tobias JH, Vernon K, Blom AW. 90 day mortality after 409,096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis, Lancet. 2013 Sep 28;382(9898):1097-104
- Premier-IHI Integrated Care Pathway for Total Joint Arthroplasty (April 2013)
- Williams-Russo P, Sharrock NE, Haas SB, et al. Randomized Trial of Epidural Versus General Anesthesia: Outcomes After Primary Total Knee Replacement. Clinical Orthopaedics & Related Research. 331:199-208, October, 1996.
- National Surgical Quality Improvement Project database
- FORCE-Total Joint Registry database
- Warren, F., Sundaram, A., Anis, A., Kamath, S., Mont, S., Higuera, S., & Piuzzi, S. (2020). Spinal Anesthesia Is Associated With Decreased Complications After Total Knee and Hip Arthroplasty. Journal of the American Academy of Orthopaedic Surgeons, 28(5), e213–e221.
CPT® only copyright 2022 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.