Transmission Data Processing Flow TJC
Version 2021B2
Joint Commission Clinical Data Processing Flow
Introduction
This section contains information regarding the order in which the Joint Commission recommends processing the Joint Commission’s national quality core measures. However, no support or further suggestion is provided on each hospital’s processing system.
The data processing flow ensures that only valid data are used in the measure algorithms. Each case that fails to pass the data validation performed by the process as recommended in this document should not be evaluated against the measure algorithm.
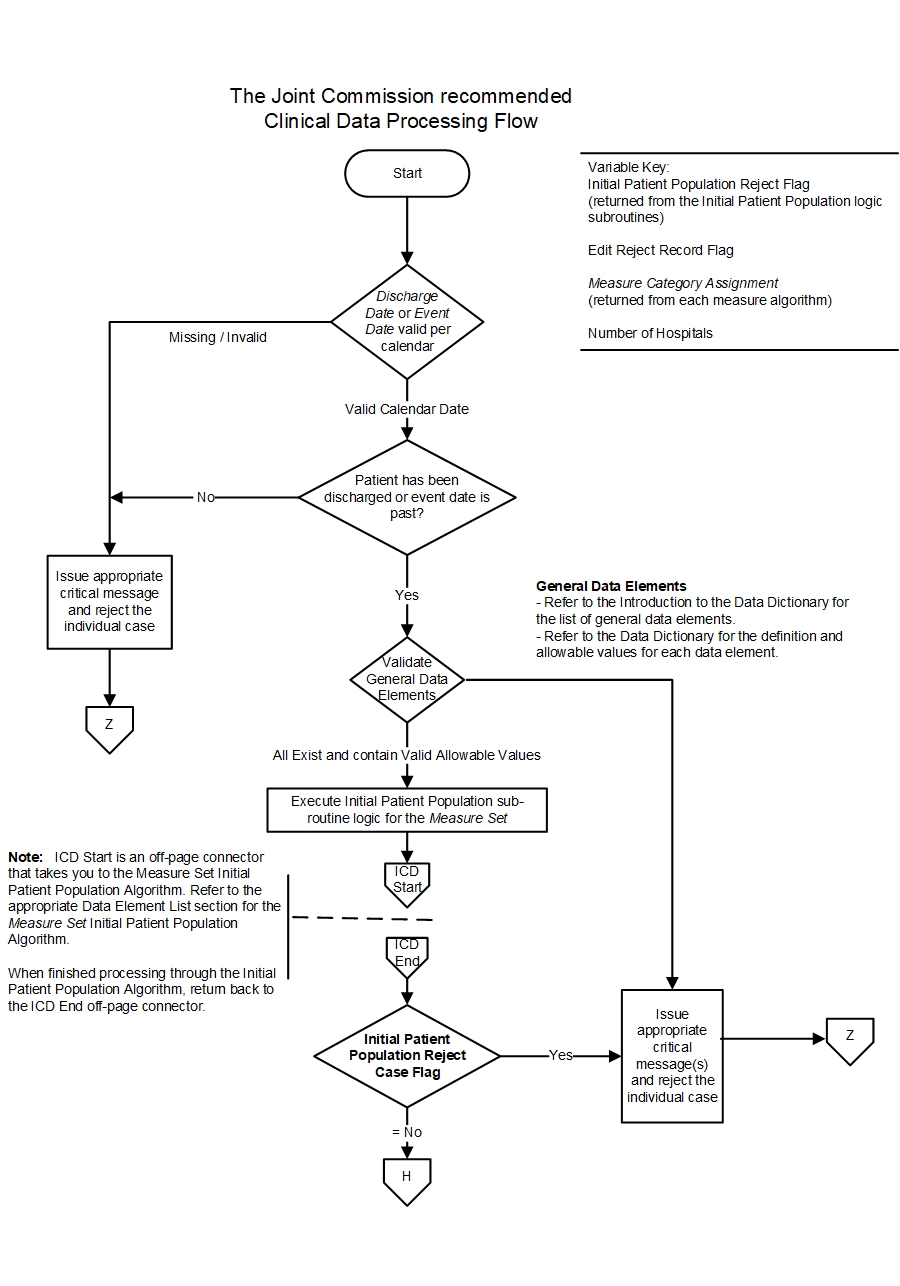
Data Processing Flow for the Joint Commission
Note HBIPS:HBIPS contains two Initial Patient Populations, discharges and events. All events of the same type occurring on the same day are reported as one measure (i.e., added as total). However, different types of events occurring on the same day are reported as different measures (i.e., different numbers). Note PC:
The PC measure set contains 2 sub-populations, PC-Mother and PC-Newborn. Cases that meet the PC-Mother sub-population will be reported for the PC-01, and PC-02 measures. All cases that meet the PC-Newborn sub-population should be reported for the PC-06 measure. The cases that meet the PC-Newborn sub-population criteria may be sampled and reported for PC-05 measure. Note CSTK:
Cases reported for the CSTK-05 measure are expected to be reported for the CSTK-02 and CSTK-10 measures: CSTK-02 (TSC Certification Only) and CSTK-10 (CSC Certification Only). The case should be followed-up within 75-105 days after the discharge date and reported for the month that the follow-up happened, corresponding with the Modified Rankin Score (MRS) Date. All processed data should have gone through the following steps:
- Data are evaluated to ensure patient has been discharged or the event date has passed and the patient’s record is complete.
- The general data elements, as defined in the Introduction to the Data Dictionary section, should be evaluated to ensure they exist and contain valid allowable values. The HBIPS measure set is unique in that it has three different groups of general data elements. The first group is "general" for all measures in the set. The second group is only "general" for the HBIPS discharge measures. The third group is only "general" for the HBIPS event measures. In addition, HBIPS data elements may be "measure set specific" for one type of HBIPS measure and "general" for the other type. For example, Psychiatric Care Setting is a "measure set specific" data element for the discharge measures and a "general" data element for the event measures. See #4 for information concerning the processing of "measure set specific" data elements.
- If any general data elements fall outside of the data integrity checks, the system should reject the case and stop processing it.
- If any general data element is missing or invalid, the system should reject the case and stop processing it.
- If all general data elements exist and contain valid allowable values, the system should continue processing the case further for measure evaluation.
- The Initial Patient Population Algorithm associated to the Measure Set is evaluated to ensure that the data is in the population of the set. Refer to the appropriate Measure Set Data Element List for the algorithm.
- If the Initial Patient Population Algorithm returns an Initial Patient Population Reject Case Flag = “Yes” (case is not in the Initial Patient Population), reject the case and stop processing.
- If the Initial Patient Population Algorithm returns an Initial Patient Population Reject Case Flag = “No” (case is in the Initial Patient Population), continue processing.
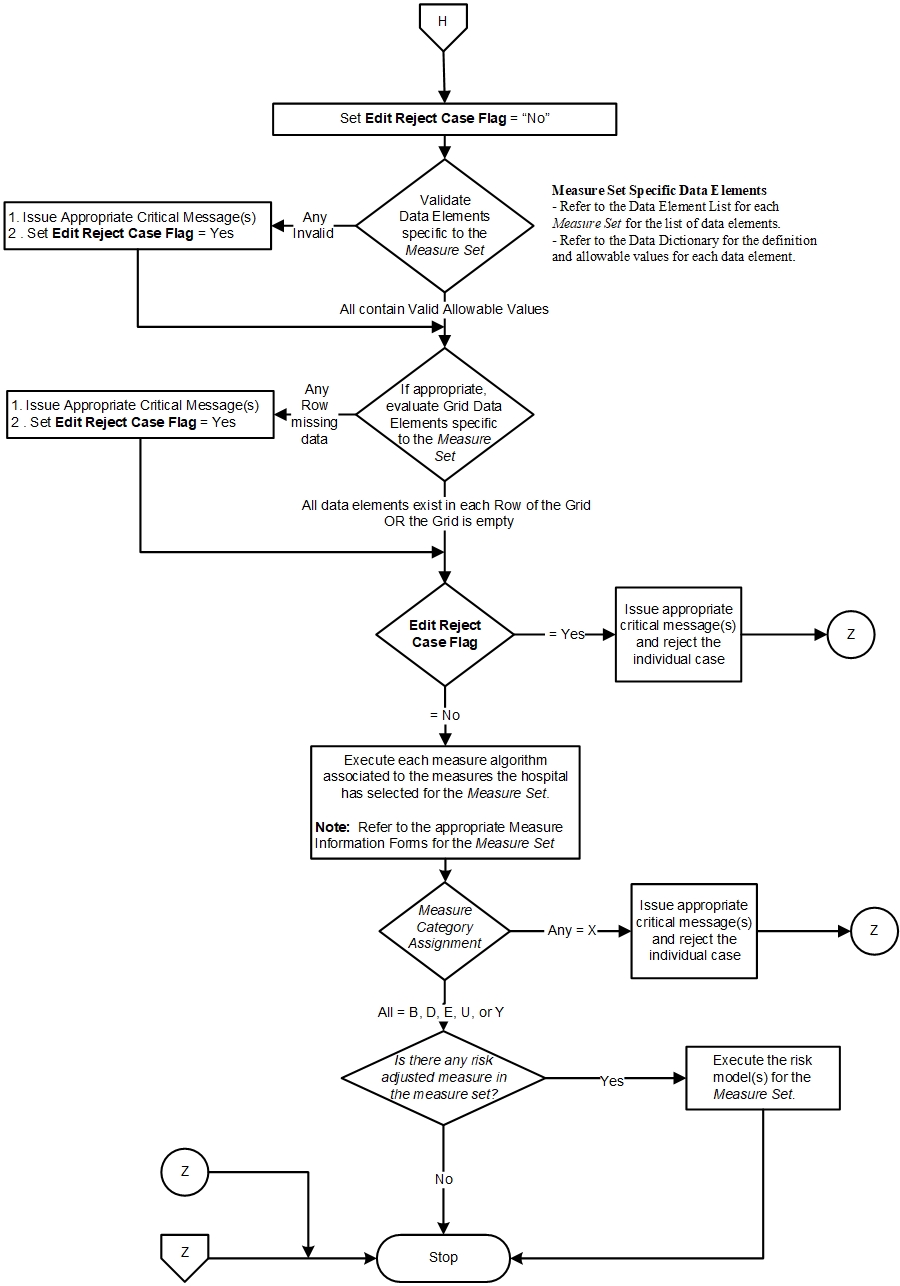
- The Measure Set specific data elements are evaluated to ensure they contain valid allowable values.
- If any of the measure set specific data elements fall outside of the data integrity checks, the system should reject the case and stop processing it.
- If any of the measure set specific data elements are invalid, the system should reject the case and stop processing it.
- If all measure set specific data elements contain valid allowable values, the system should continue processing the case further for measure evaluation.
- If appropriate for the Measure Set, grid data elements are evaluated to ensure each row does not contain missing data.
- If any row of the grid is missing data, the system should reject the case and stop processing it.
- If the grid is empty or all data elements exist in each row, continue processing.
- Execute the algorithm for the measures the hospital will be reporting.
- If any measure for the case evaluates with a Measure Category Assignment = “X”, “reject” the case and do not use it to aggregate the final measure rate.
- If all measures for the case evaluate with Measure Category Assignments = “B”, “D”, “E”, "U", and/or “Y”, include the case in the aggregation of each measure’s rate.
- Before aggregating each measure’s monthly data, ensure that the month or quarter is closed, depending on your hospital’s sampling methodology, and all patients for that time period has been processed through the above steps.
Clinical Data Processing Flow
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.