Measure Information Form
Version 2021B2
Measure Information Form
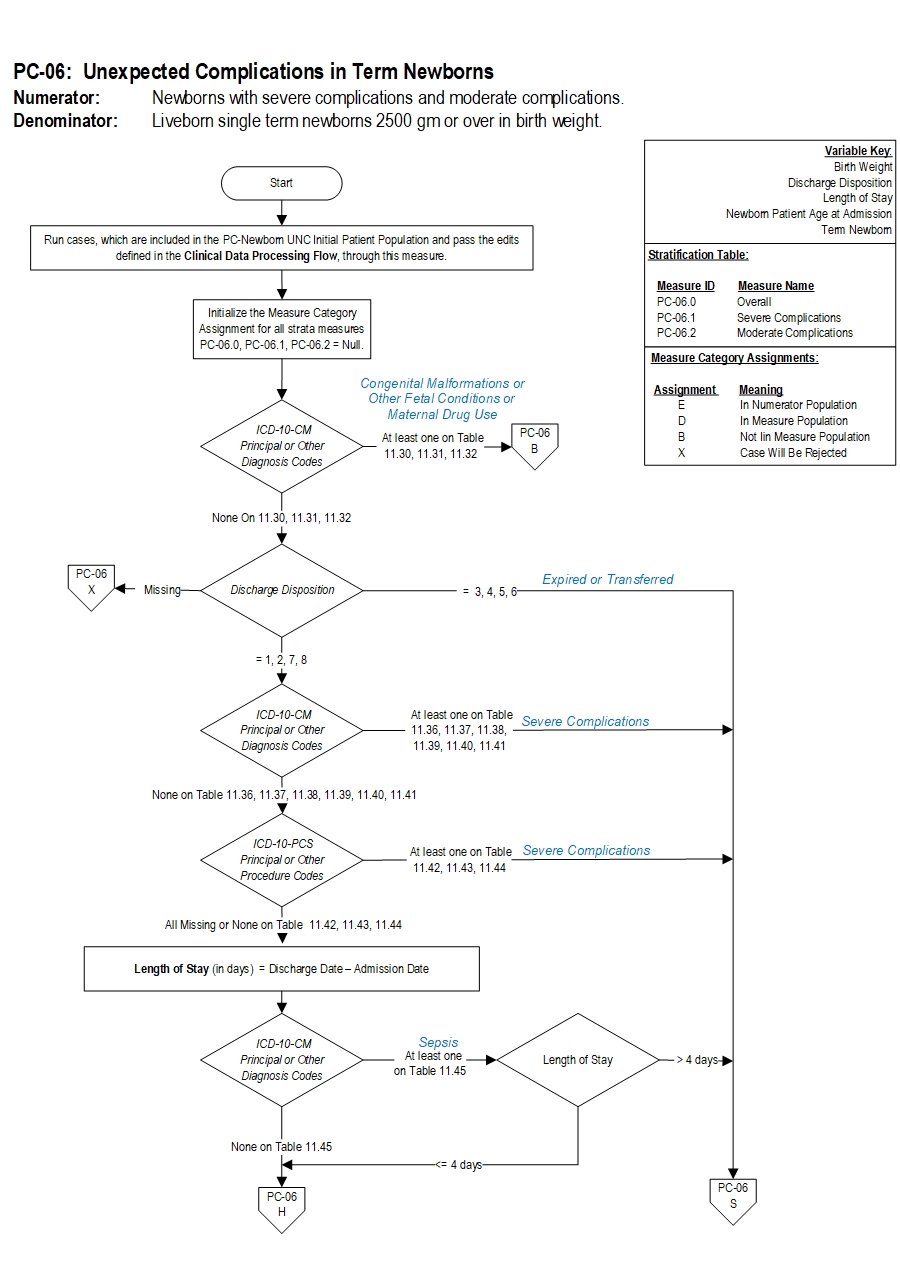
Severe complications include neonatal death, transfer to another hospital for higher level of care, severe birth injuries such as intracranial hemorrhage or nerve injury, neurologic damage, severe respiratory and infectious complications such as sepsis.
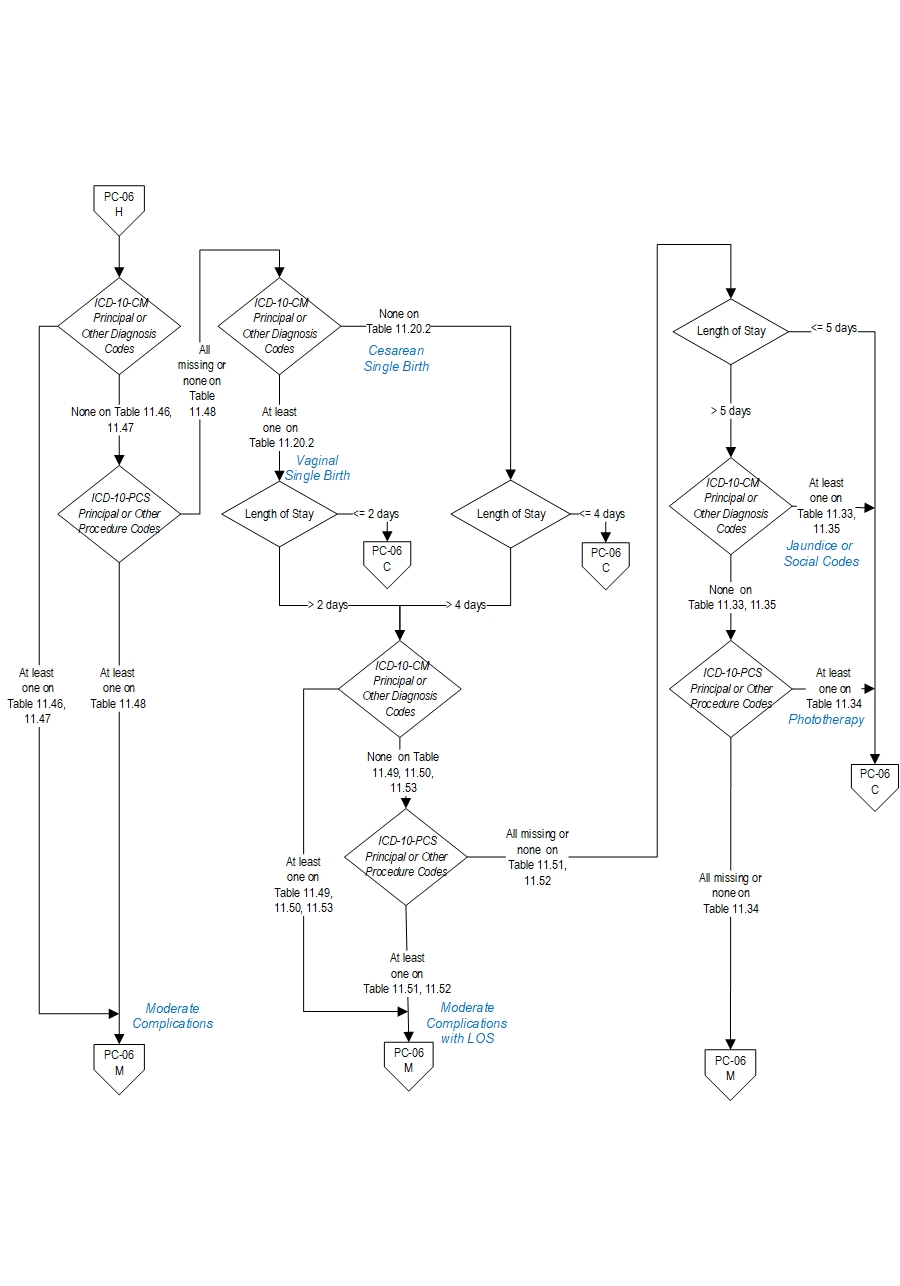
Moderate complications include diagnoses or procedures that raise concern but at a lower level than the list for severe e.g. use of CPAP or bone fracture. Examples include less severe respiratory complications e.g. Transient Tachypnea of the Newborn, or infections with a longer length of stay not including sepsis, infants who have a prolonged length of stay of over 5 days.
Rationale: The most important childbirth outcome for families is bringing home a healthy baby. While there have been measures developed to assess clinical practices and outcomes in preterm infants, there is a lack of metrics that assess the health outcomes of term infants who represent over 90% of all births. This measure addresses this gap and gauges adverse outcomes resulting in severe or moderate morbidity in otherwise healthy term infants without preexisting conditions. This measure also uses length of stay (LOS) modifiers to guard against overcoding and undercoding of diagnoses. Importantly, this metric also serves as a balancing measure for other maternal measures such as NTSV Cesarean rates and early elective delivery rates. The purpose of a balancing measure is to guard against any unanticipated or unintended consequences of quality improvement activities for these measures.Type Of Measure: Outcome Improvement Noted As: Decrease in the rate
PC-06.0 Newborns with severe complications and moderate complications.
PC-06.1 Newborns with severe complications.
PC-06.2 Newborns with moderate complications.
Included Populations:Denominator Statement: Liveborn single term newborns 2500 gm or over in birth weight.
Severe Complications:Moderate Complications:
- Death
- Transfer to another acute care facility for higher level of care
- ICD-10-CM Principal Diagnosis Code, ICD-10-CM Other Diagnosis Codes, ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for Severe Morbidities as defined in Appendix A, Tables:
- 11.36 Severe Birth Trauma
- 11.37 Severe Hypoxia/Asphyxia
- 11.38 Severe Shock and Resuscitation
- 11.39 Neonatal Severe Respiratory Complications
- 11.40 Neonatal Severe Infection
- 11.41 Neonatal Severe Neurological Complications
- 11.42 Severe Shock and Resuscitation Procedures
- 11.43 Neonatal Severe Respiratory Procedures
- 11.44 Neonatal Severe Neurological Procedures
- Patients with Length of Stay greater than 4 days AND an ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for Sepsis as defined in Appendix A, Table 11.45 Neonatal Severe Septicemia
Excluded Populations: None Data Elements:
- ICD-10-CM Principal Diagnosis Code, ICD-10-CM Other Diagnosis Codes, ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for moderate complications as defined in Appendix A, Tables:
- 11.46 Moderate Birth Trauma
- 11.47 Moderate Respiratory Complications
- 11.48 Moderate Respiratory Complications Procedures
- ICD-10-CM Principal Diagnosis Code for single liveborn newborn as defined in Appendix A, Table 11.20.2 Single Liveborn Newborn-Vaginal AND Length of Stay greater than 2 days
OR
ICD-10-CM Principal Diagnosis Code for single liveborn newborn as defined in Appendix A, Table 11.20.3 Single Liveborn Newborn-Cesarean AND Length of Stay greater than 4 days
AND ANY
ICD-10-CM Principal Diagnosis Code, ICD-10-CM Other Diagnosis Codes, ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for moderate complications as defined in Appendix A, Tables:
- 11.49 Moderate Birth Trauma with LOS
- 11.50 Moderate Respiratory Complications with LOS
- 11.51 Moderate Neurological Complications with LOS Procedures
- 11.52 Moderate Respiratory Complications with LOS Procedures
- 11.53 Moderate Infection with LOS
- Patients with Length of Stay greater than 5 days and NO ICD-10-CM Principal Diagnosis Code, ICD-10-CM Other Diagnosis Codes, ICD-10-PCS Principal Procedure Code or ICD-10-PCS Other Procedure Codes for jaundice or social indications as defined in Appendix A, Tables:
- 11.33 Neonatal Jaundice
- 11.34 Phototherapy
- 11.35 Social Indications
Included Populations: Single liveborn newborns with ICD-10-CM Principal Diagnosis Code for single liveborn newborn as defined in Appendix A, Table Number 11.20.1: Single Liveborn Newborn Excluded Populations:Data Elements:
- Patients who are not born in the hospital or are part of multiple gestation pregnancies, with no ICD-10-CM Principal Diagnosis Code for single liveborn newborn as defined in Appendix A, Table Number 11.20.1: Single Liveborn Newborn
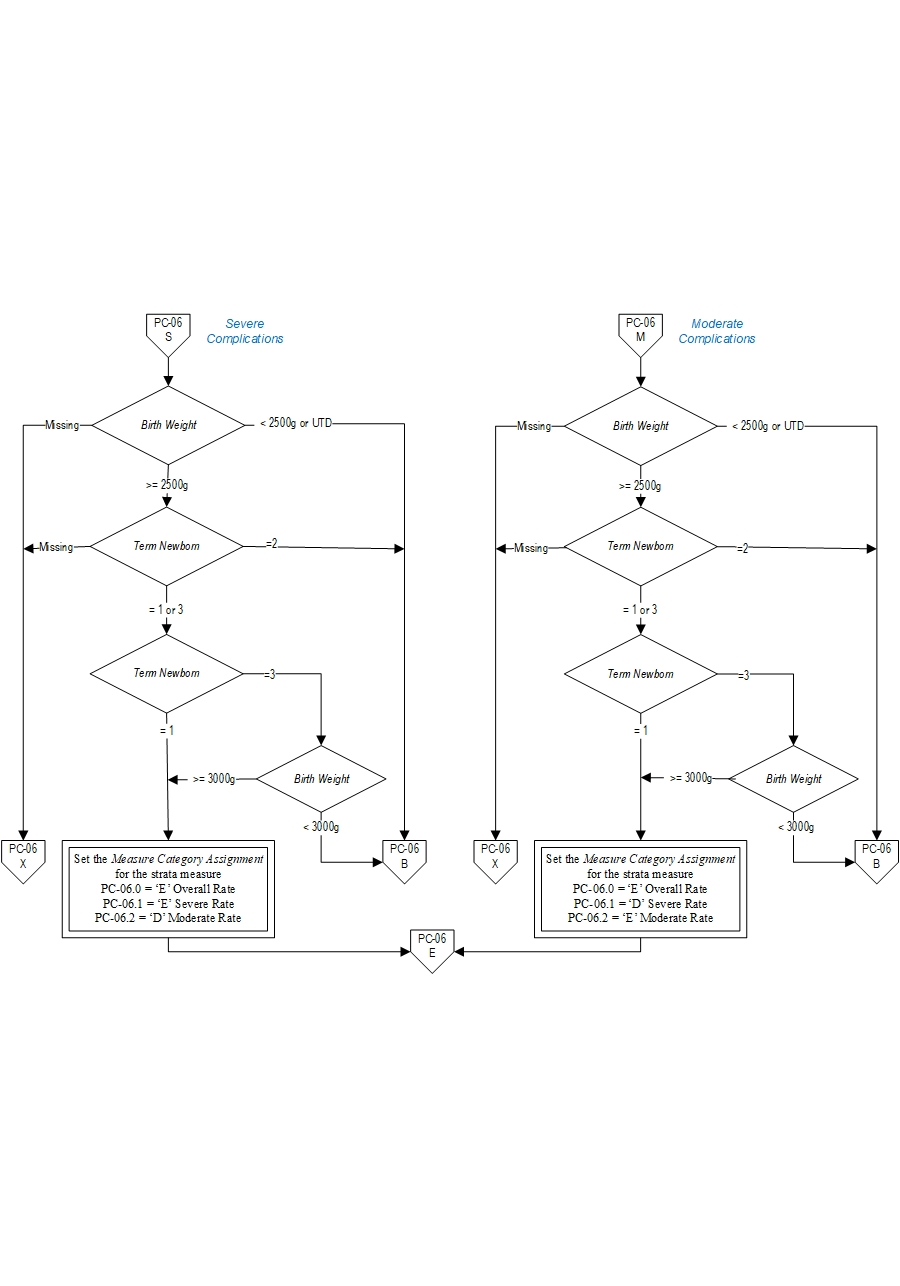
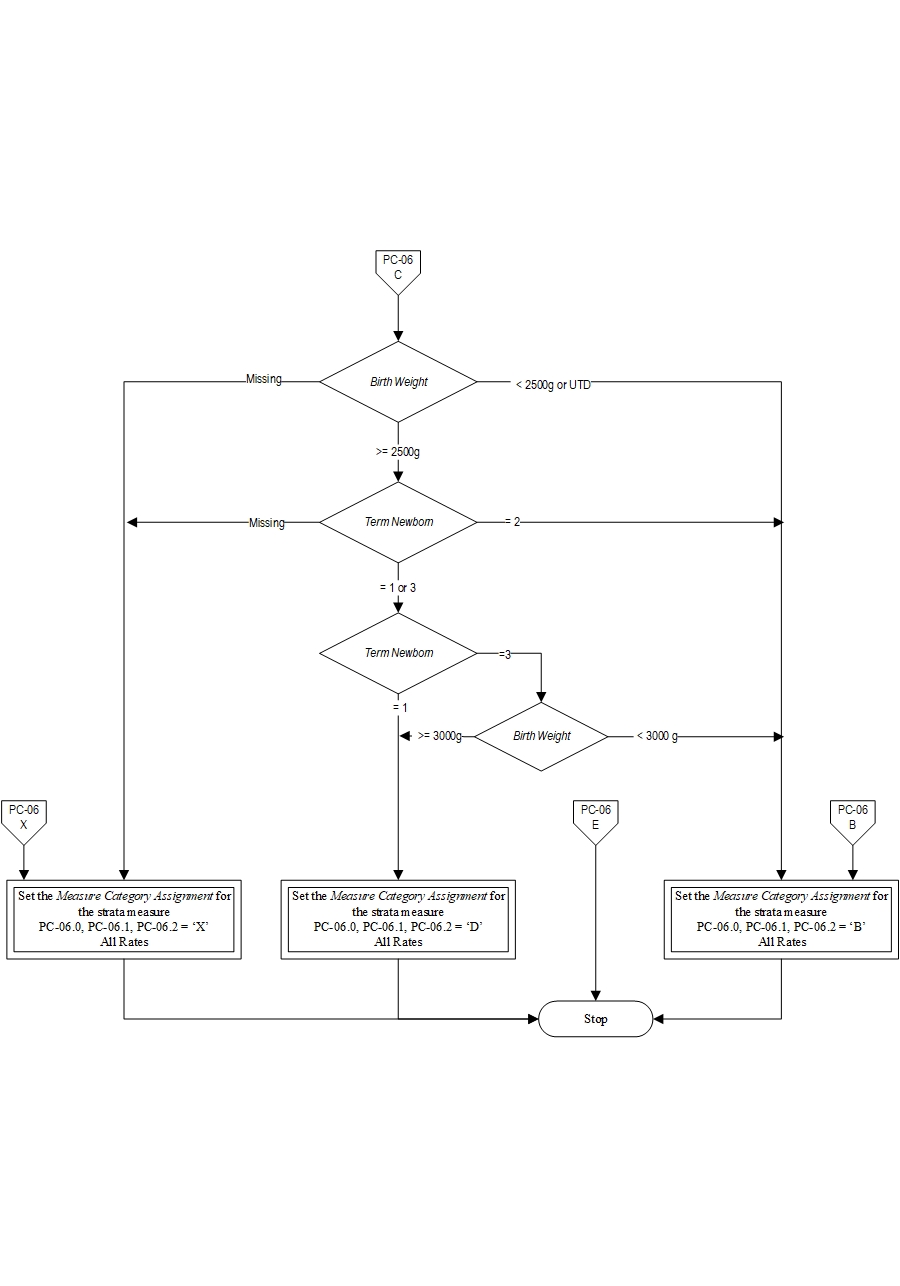
- Birth Weight < 2500 gm
- Patients who are not term or with < 37 weeks gestation completed
- Patients whose term status or gestational age is missing and birthweight < 3000 gm
- ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for congenital malformations and genetic diseases as defined in Appendix A, Table 11.30 Congenital Malformations
- ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for pre-existing fetal conditions as defined in Appendix A, Table 11.31 Fetal Conditions
- ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Codes for maternal drug use exposure in-utero as defined in Appendix A, Table 11.32 Maternal Drug Use
Note:
All 3 sub-measures will have the same Final Denominator.
Final Denominator = Number of patients in Overall Numerator (PC-06.0=category E) + Number of cases in Overall Denominator (PC-06.0= category of D) Rate Calculation:
PC-06.0: Overall rate = ((Number of patients with Severe Complications + Number of patients with Moderate Complications) / Final Denominator) * 1000
PC-06.1: Severe rate = (Number of patients with Severe Complications / Final Denominator) * 1000
PC-06.2: Moderate rate = (Number of patients with Moderate Complications / Final Denominator) * 1000
Selected References:
- Martin JA, Hamilton BE, Ventura SJ et al. Births: Final data for 2010. National vital statistics reports; vol 61 no1. Hyattsville, MD: National Center for health Statistics. 2012
- Russo, C. A (Thomson Reuters) and Andrews, R.M (AHRQ). Potentially Avoidable Injuries to mothers and Newborns During Childbirth, 2006. HCUP Statistical Brief # 74. June 2009. Agency for Healthcare Research and Quality. Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statsbriefs/sb74.pdf.
- Gregory KD, Fridman M, Shah S et al. Global measures of quality and patient safety-related childbirth outcomes: should we monitor adverse or ideal rates? Am J Obstet Gynecol 2009;200:681.e1-681.e7.
- Profit J, Zupancic JA, Gould JB et al. Implementing pay-for-performance in the neonatal intensive care unit. Pediatrics 2007;119:975-82
- Operative Vaginal Delivery. Practice Bulletin No. 17. American College of Obstetricians and Gynecologists. Obstet Gynecol 2000;1-8
- Shoulder dystocia. ACOG Practice Bulletin No. 40. American College of Obstetrician and Gynecologists. Obstet Gynecol 2002;100:1045-50
- Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease. MMWR 2010;59 (No. RR 10): 1-36 http://www.cdc.gov/mmwr/pdf/rr/rr5910.pdf
- Wilmink FA, Hukkelhoven CW, Lunshof, S et al. Neonatal outcome following elective cesarean section beyond 37 weeks of gestation: a 7- year retrospective analysis of a national registry.2010 Am J Obstet Gynecol 2002:250.e1-8
- Tita AT, Landon MB, Spong CY et al. Timing of Elective Repeat Cesarean Delivery at Term and Neonatal Outcomes. N Engl J Med 2009;360(2):111-20
- Hansen AK, Wisborg K, Uldbjerg N. Risk of respiratory morbidity in term infants delivered by elective cesarean section: cohort study. BMJ 2008;336:85
- Spong CY. Defining “Term” pregnancy: Recommendations from the defining “term” pregnancy workgroup. JAMA. 2013 Jun 19;309(23):2445-6.
- Zhang X and Kramer MS. Variations in Mortality and Morbidity by Gestational Age among Infants Born at Term. J Pediatr 2009;154:358-62
- Fleischman AR, Oinuma M and Clark SL. Rethinking the Definition of “Term Pregnancy”. Obstet Gynecol 2010;116(1)136-139
- Clark SL, Miller DD, Belfort MA, et al. Neonatal and maternal outcomes associated with elective term delivery. Am J Obstet Gynecol 2009;200:156.e1-156.e4
- Reddy UM, Bettegowda VR, Dias T et al. Term Pregnancy: A period of Heterogeneous Risk for Infant Mortality. Obstet Gynecol 2011;117:1279-1287
- "Unexpected Complications in Term Newborns.” California Maternal Quality Care Collaborative (CMQCC), 2018, www.cmqcc.org/focus-areas/quality-metrics/unexpected-complications-term-newborns.
California Maternal Quality Care Collaborative
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.