Measure Information Form
Version 2021B2
Measure Information Form
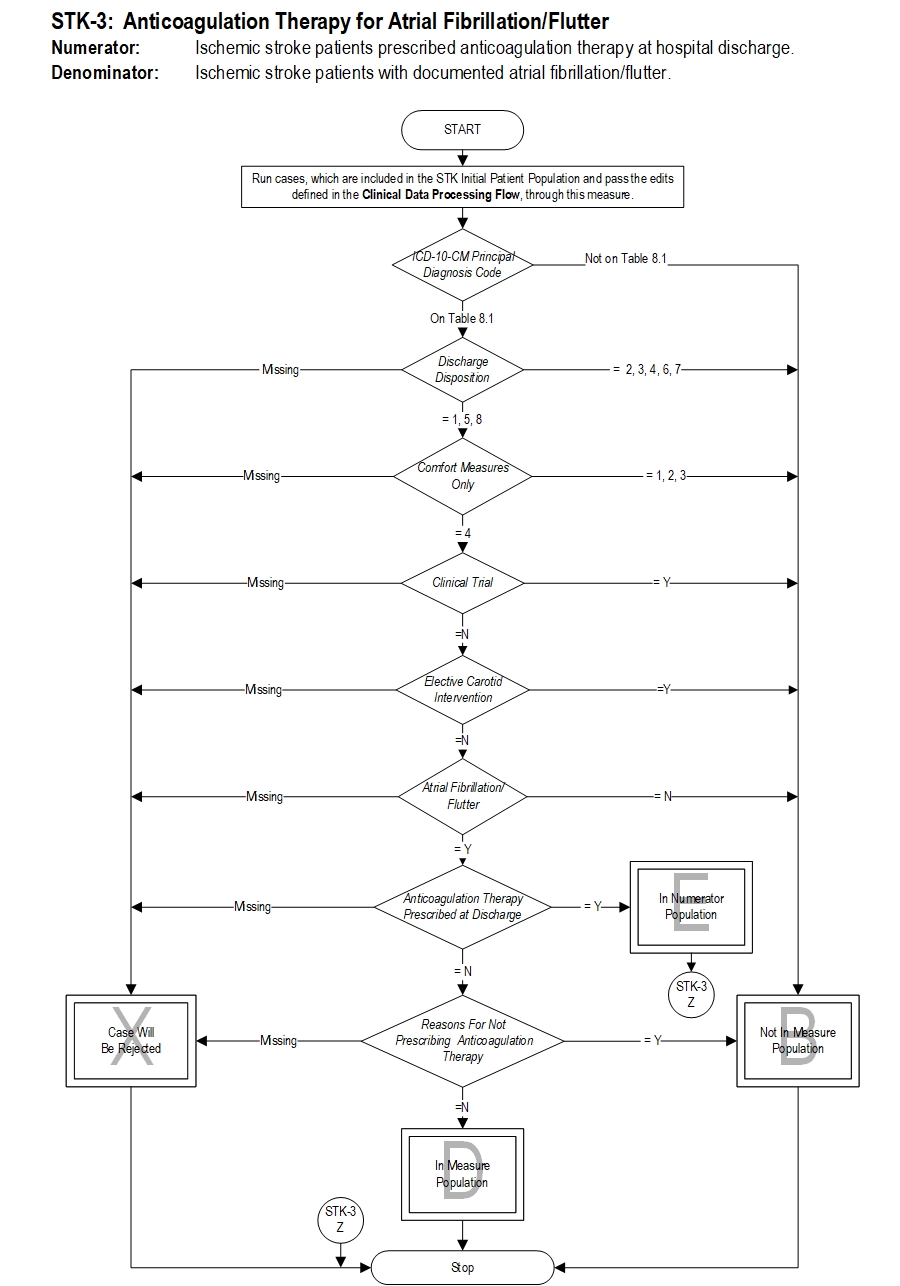
Included Populations: Not applicable Excluded Populations: None Data Elements:Denominator Statement: Ischemic stroke patients with documented atrial fibrillation/flutter.
Included Populations:Excluded Populations:
- Discharges with an ICD-10-CM Principal Diagnosis Code for ischemic stroke as defined in Appendix A, Table 8.1
- Patients with documented Atrial Fibrillation/Flutter
Data Elements:
- Patients less than 18 years of age
- Patients who have a Length of Stay greater than 120 days
- Patients with Comfort Measures Only documented
- Patients enrolled in clinical trials
- Patients admitted for Elective Carotid Intervention
- Patients discharged to another hospital
- Patients who left against medical advice
- Patients who expired
- Patients discharged to home for hospice care
- Patients discharged to a health care facility for hospice care
- Patients with a documented Reason For Not Prescribing Anticoagulation Therapy
- Berge, E., M. Abdelnoor, P. H. Nakstad, and P. M. Sandset. "Low Molecular-Weight Heparin Versus Aspirin in Patients with Acute Ischaemic Stroke and Atrial Fibrillation: A Double-Blind Randomised Study. Haest Study Group. Heparin in Acute Embolic Stroke Trial." [In eng]. Lancet 355, no. 9211 (Apr 8 2000): 1205-10.
- Centers for Disease Control and Prevention. "Prevalence and Most Common Causes of Disability among Adults--United States, 2005." [In eng]. MMWR Morb Mortal Wkly Rep 58, no. 16 (May 1 2009): 421-6.
- Connolly, S. J., M. D. Ezekowitz, S. Yusuf, J. Eikelboom, J. Oldgren, A. Parekh, J. Pogue, et al. "Dabigatran Versus Warfarin in Patients with Atrial Fibrillation." [In eng]. N Engl J Med 361, no. 12 (Sep 17 2009): 1139-51.
- Fuster, V., L. E. Ryden, R. W. Asinger, D. S. Cannom, H. J. Crijns, R. L. Frye, J. L. Halperin, et al. "Acc/Aha/Esc Guidelines for the Management of Patients with Atrial Fibrillation: Executive Summary. A Report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation): Developed in Collaboration with the North American Society of Pacing and Electrophysiology." [In eng]. J Am Coll Cardiol 38, no. 4 (Oct 2001): 1231-66.
- Fuster, V., L. E. Ryden, D. S. Cannom, H. J. Crijns, A. B. Curtis, K. A. Ellenbogen, J. L. Halperin, et al. "Acc/Aha/Esc 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): Developed in Collaboration with the European Heart Rhythm Association and the Heart Rhythm Society." [In eng]. Circulation 114, no. 7 (Aug 15 2006): e257-354.
- Goldstein, L. B., R. Adams, M. J. Alberts, L. J. Appel, L. M. Brass, C. D. Bushnell, A. Culebras, et al. "Primary Prevention of Ischemic Stroke: A Guideline from the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology Affirms the Value of This Guideline." [In eng]. Stroke 37, no. 6 (Jun 2006): 1583-633.
- Gorelick, P. B., R. L. Sacco, D. B. Smith, M. Alberts, L. Mustone-Alexander, D. Rader, J. L. Ross, et al. "Prevention of a First Stroke: A Review of Guidelines and a Multidisciplinary Consensus Statement from the National Stroke Association." [In eng]. JAMA 281, no. 12 (Mar 24-31 1999): 1112-20.
- Hart, R. G., O. Benavente, R. McBride, and L. A. Pearce. "Antithrombotic Therapy to Prevent Stroke in Patients with Atrial Fibrillation: A Meta-Analysis." [In eng]. Ann Intern Med 131, no. 7 (Oct 5 1999): 492-501.
- January, C.T., Wann, S., Calkins, H., Chen, L.Y., Cigarroa, J.E., Cleveland, J.C., Ellinor, P.T., et al. "Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society…2019 AHA/ACC/HRS Focused Update of the 2014 AHA/...A Report of the American College of Cardiology/American Heart Association." [In eng.]. Circulation 140, no. 2 (Jan 28 2019):e125–e151.
- Jauch, E. C., J. L. Saver, H. P. Adams, Jr., A. Bruno, J. J. Connors, B. M. Demaerschalk, P. Khatri, et al. "Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association." [In Eng]. Stroke (Jan 31 2013).
- Kernan, W.N., B. Ovbiagele, H. R. Black, D. M. Bravata, M. I. Chimowitz, M. D. Ezekowitz, M. C. Fang, M. Fisher, K. L. Furie, D. V. Heck, S. C. Johnston, S. E. Kasner, S. J. Kittner, P. H. Mitchell, M. W. Rich, D. Richardson, L. H. Schwamm, J. A. Wilson. “Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association.” [in eng.] Stroke 45, no. 7 (May 2014): 2160-223.
- Lin, H. J., P. A. Wolf, M. Kelly-Hayes, A. S. Beiser, C. S. Kase, E. J. Benjamin, and R. B. D'Agostino. "Stroke Severity in Atrial Fibrillation. The Framingham Study." [In eng]. Stroke 27, no. 10 (Oct 1996): 1760-4.
- Penado, S., M. Cano, O. Acha, J. L. Hernandez, and J. A. Riancho. "Atrial Fibrillation as a Risk Factor for Stroke Recurrence." [In eng]. Am J Med 114, no. 3 (Feb 15 2003): 206-10.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, et al; on behalf of the American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018 Jan;49:e31-e32.
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke. A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418.
- Roger, V. L., A. S. Go, D. M. Lloyd-Jones, E. J. Benjamin, J. D. Berry, W. B. Borden, D. M. Bravata, et al. "Heart Disease and Stroke Statistics--2012 Update: A Report from the American Heart Association." [In eng]. Circulation 125, no. 1 (Jan 3 2012): e2-e220.
- Saxena, R., and P. J. Koudstaal. "Anticoagulants for Preventing Stroke in Patients with Nonrheumatic Atrial Fibrillation and a History of Stroke or Transient Ischemic Attack (Review). ." Cochrane Database Syst Rev, no. 4 (2011): CD000185.
- Saxena, R., S. Lewis, E. Berge, P. A. Sandercock, and P. J. Koudstaal. "Risk of Early Death and Recurrent Stroke and Effect of Heparin in 3169 Patients with Acute Ischemic Stroke and Atrial Fibrillation in the International Stroke Trial." [In eng]. Stroke 32, no. 10 (Oct 2001): 2333-7.
- van Walraven, C., R. G. Hart, D. E. Singer, A. Laupacis, S. Connolly, P. Petersen, P. J. Koudstaal, Y. Chang, and B. Hellemons. "Oral Anticoagulants Vs Aspirin in Nonvalvular Atrial Fibrillation: An Individual Patient Meta-Analysis." [In eng]. JAMA 288, no. 19 (Nov 20 2002): 2441-8.
- Wann, L. S., A. B. Curtis, K. A. Ellenbogen, N. A. Estes, 3rd, M. D. Ezekowitz, W. M. Jackman, C. T. January, et al. "2011 Accf/Aha/Hrs Focused Update on the Management of Patients with Atrial Fibrillation (Update on Dabigatran): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines." [In eng]. J Am Coll Cardiol 57, no. 11 (Mar 15 2011): 1330-7.
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.