Measure Information Form
Version 2021A
Measure Information Form
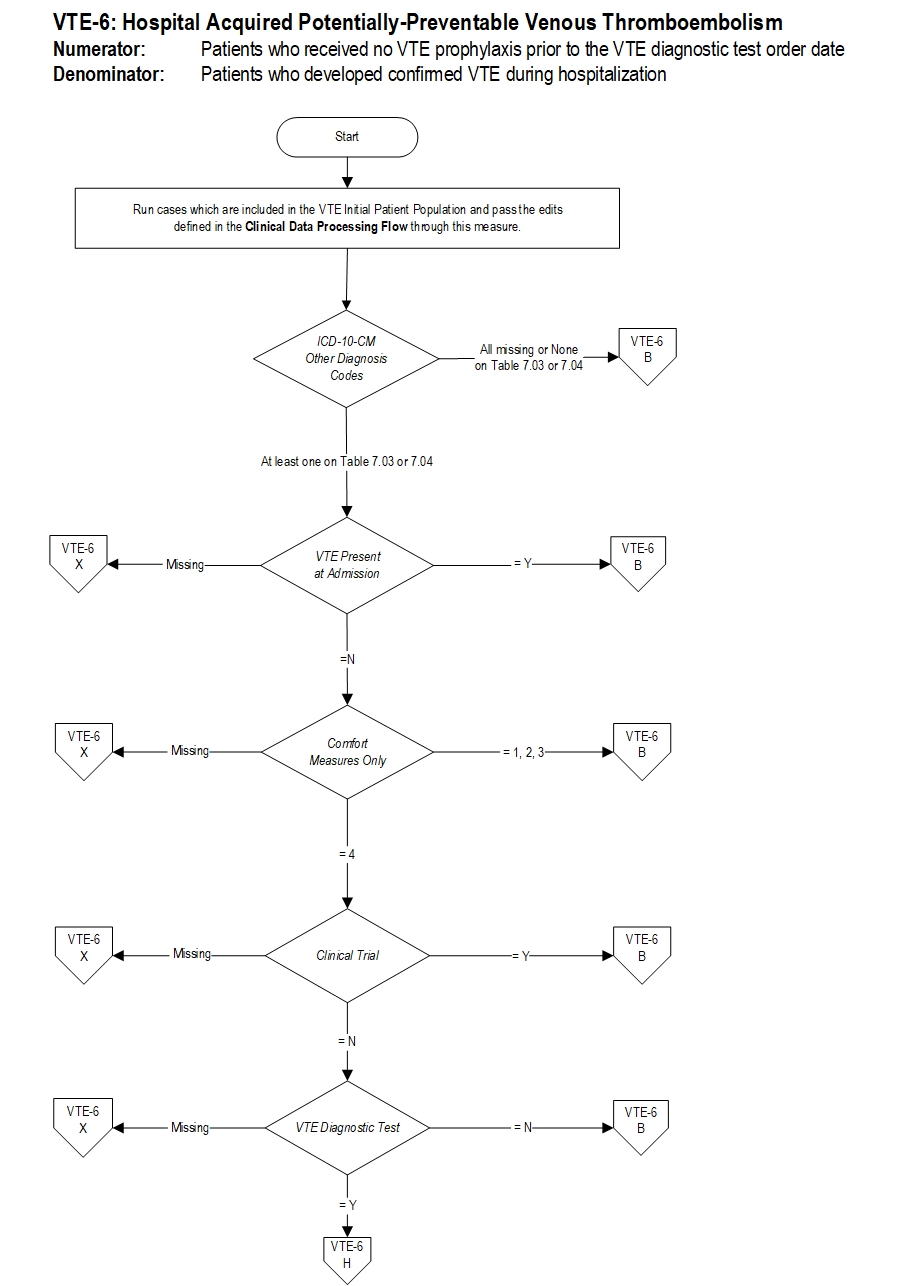
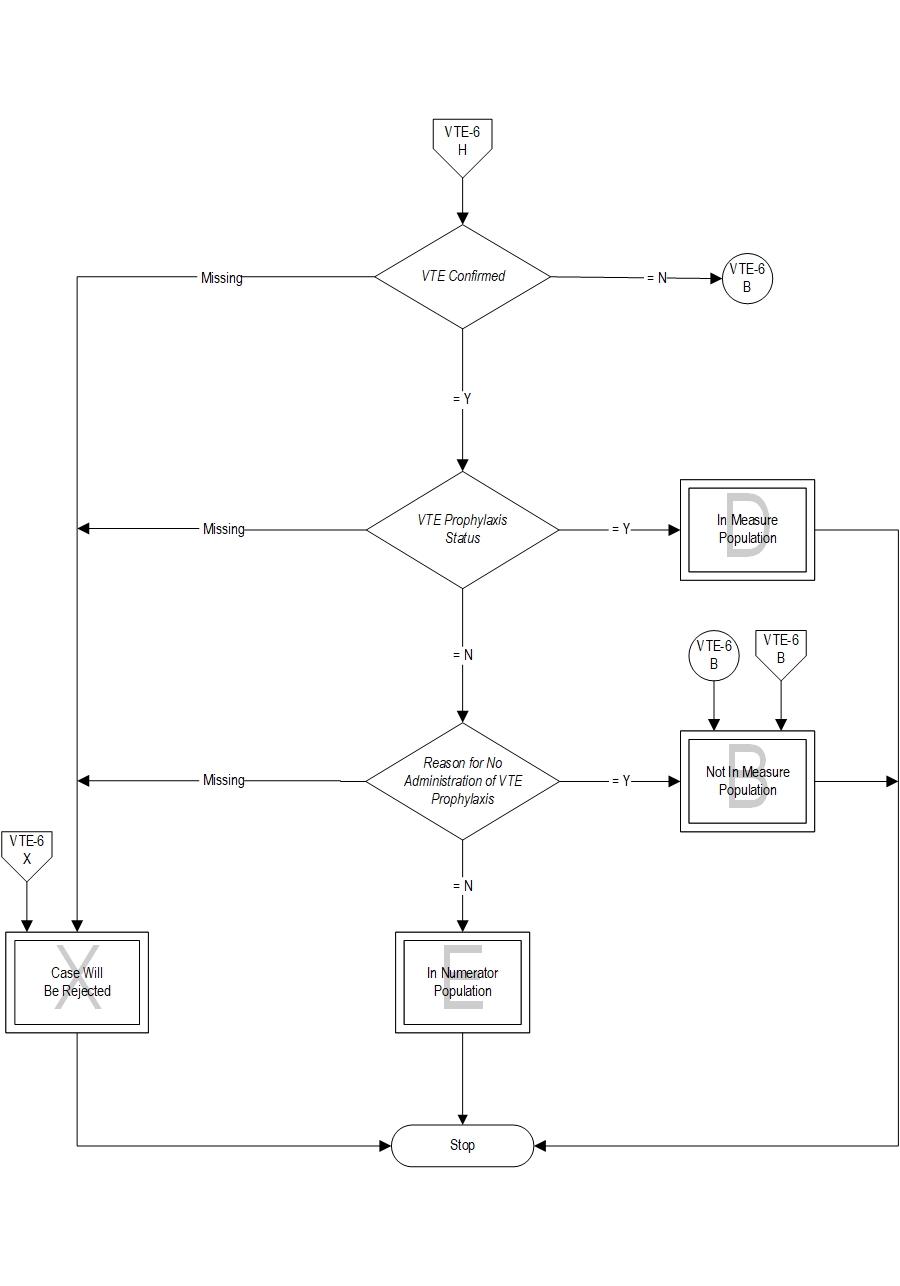
Included Populations: Not applicable Excluded Populations: None Data Elements:Denominator Statement: Patients who developed confirmed VTE during hospitalization.
Included Populations: Discharges with an ICD-10-CM Other Diagnosis Codes of VTE as defined in Appendix A, Table 7.03 or 7.04 Excluded Populations:Data Elements:
- Patients less than 18 years of age
- Patients who have a length of stay greater than 120 days
- Patients with Comfort Measures Only documented
- Patients enrolled in clinical trials
- Patients with ICD-10-CM Principal Diagnosis Code of VTE as defined in Appendix A, Table 7.03 or 7.04
- Patients with VTE Present at Admission
- Patients with reasons for not administering mechanical and pharmacologic prophylaxis
- Patients without VTE confirmed by diagnostic testing
- Anderson DR, Morgano GP, Bennett C, Dentali F, Francis CW, Garcia DA, et. al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Advances. 2019, Dec;3(23):3898-3944.
- Arnold DM, Kahn SR, Shrier I. Missed opportunities for prevention of venous thromboembolism: an evaluation of the use of thromboprophylaxis guidelines. Chest. 2001 Dec;120(6):1964-71.
- Baglin TP, White K, Charles A. Fatal pulmonary embolism in hospitalized medical patients. J Clin Pathol. 1997 Jul;50(7):609-10.
- Dupras D, Bluhm J, Felty C, Hansen C, Johnson T, Lim K, Maddali S, Marshall P, Messner P, Skeik N. Venous thromboembolism diagnosis and treatment. Bloomington (MN): Institute for Clinical Systems Improvement (ICSI); 2013 Jan. 90 p.
- Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism. The Eighth ACCP Conference on antithrombotic and thrombolytic therapy. Chest. 2008; 133:381S-453S.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004 Sep;126(3 Suppl):338S-400S.
- Gillies TE, Ruckley CV, Nixon SJ. Still missing the boat with fatal pulmonary embolism. Br J Surg. 1996 Oct;83(10):1394-5.
- Goldhaber SZ, Dunn K, Mac Dougall RC. New onset of venous thromboembolism among hospitalized patients at Brigham and Women’s Hospital is caused more often by prophylaxis failure than by withholding treatment. Chest 2000;118:1680
- Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 Suppl):e227S-77S.
- Guyatt, GH, Akl, EA, Crowther, M, Gutterman, D, Schunemann, H Antithromboitic Therapy and Prevention of Thrombosis, 9th edition: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(2)(Supp):7S-47S.
- Heit JA, Cohen AT, Anderson FA Jr, et al. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US, Blood (ASH Annual Meeting Abstracts), 2005;106:Abstract 910.
- Heit JA, O'Fallon WM, Petterson TM et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002 Jun 10;162(11):1245-8.
- ISTH Steering Committee for World Thrombosis Day. “Thrombosis: A Major Contributor to Global Disease Burden.” Thrombosis Research, vol. 134, no. 5, 2014, pp. 931-938.
- Jobin S, Kalliainen L, Adebayo L, Agarwal Z, Card R, Christie B, Haland T, Hartmark M, Johnson P, Kang M, Lindvall B, Mohsin S, Morton C. Venous thromboembolism prophylaxis. Bloomington (MN): Institute for Clinical Systems Improvement (ICSI); 2012 Nov. 51 p.
- Kahn S, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, and Murad MH corresponding author. "Prevention of VTE in Nonsurgical Patients Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines." Chest 141.2 Suppl (2012): e195S.
- Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012 Feb;141(2 Suppl):e419S-94S.
- Khoury L, Dangodara AA, Lee JA, Lovejoy M, Amin AN. “Implementation of a Mandated Venous Thromboembolism Clinical Order Set Improves Venous Thromboembolism Core Measures.” Hospital Practice (1995), vol. 42, no. 5, 2014, pp. 89-99.
- Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris T, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores CL, Antithrombotic Therapy for VTE Disease: CHEST Guideline, CHEST (2016), doi: 10.1016/j.chest.2015.11.026.
- Wachter R, Shojania KG, Duncan BW, McDonald KW, et al. Making health care safer: a critical analysis of patient safety practices; evidence report/ technology assessment No 43. Agency for Healthcare Research and Quality. Publication 01-E0582001.2001. Retrieved October 11, 2011 from http://archive.ahrq.gov/clinic/tp/ptsaftp.htm.
- Zhan C, Miller MR. Excessive length of stay, charges and mortality attributable to medical injures during hospitalization. JAMA 2003; 290:1868-1874.
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.