Measure Information Form
Version 2020B1
Measure Information Form
Hips: [VR-12 or PROMIS-Global] AND [HOOS Jr. (6 questions) or HOOS Pain, Function Daily Living Subscales (27 questions)]
Knees: [VR-12 or PROMIS-Global] AND [KOOS Jr. (7 questions) or KOOS Stiffness, Pain, Function Daily Living Subscales (28 questions)]
The assessment tool must reflect the status of the patient’s health condition, health behavior, or experience with health care from the patient’s perspective, without interpretation of the patient’s response by a clinician or anyone else 1. The assessment can be completed by phone, mail, email, or in-person.Note: Links to the survey instruments are provided in the Selected References at the bottom of this section.
Rationale: Good orthopedic care requires knowledge of the patient’s history of musculoskeletal pain and associated limitations in daily function. Standardized measures of patient-reported outcomes (PROs) can provide this information. Integrating PROs into routine orthopedic patient visits can provide key information to monitor changes in symptom severity over time, support shared clinical care decisions, and assess treatment effectiveness.2 Patient reported outcome measures (PROMs) capture patients’ self-assessments of their health and provide a mechanism for evaluating the effectiveness of patient-centered care.3 In acknowledgement of the administrative burden associated with PRO data capture, The Joint Commission will implement PROMs in a phased approach. During this first phase, the process of collecting preoperative data will be measured. During the second phase, pre- and postoperative data will be evaluated with the goal of calculating patients’ improvement scores. The American Academy of Orthopedic Surgeons and American Association of Hip and Knee Surgeons are very supportive of the Centers for Medicare and Medicaid Services’ effort to develop patient-reported functional status outcome measures for total hip and knee arthroplasty. When fully specified and risk-adjusted, these measures will be useful in assessing quality and value of care and will permit performance measurement progression beyond process measures.4-5 On August 31, 2015, the American Association of Hip and Knee Surgeons (AAHKS) convened a Patient Reported Outcomes Summit for Total Joint Arthroplasty in Baltimore, Maryland. Representatives from orthopaedic organizations (AAHKS, AAOS, The Hip Society, The Knee Society, and American Joint Replacement Registry), CMS, YNHHSC/CORE, National Committee for Quality Assurance (NCQA), Mathematica, CECity, and Blue Cross Blue Shield Association participated in the Summit. The Summit’s goal was to obtain a consensus regarding the patient-reported outcomes (PRO) and risk variables suitable for total hip and knee arthroplasty performance measures.6 The instruments specified in this measure, align with the Summit’s recommendations as well as CMS Comprehensive Care for Joint Replacement (CJR) legislation.The Veterans RAND 12 Item Health Survey (VR-12) is a generic patient reported outcome (PRO) instrument used to measure health related quality of life. This tool, which measures physical function and health status, is widely used and is well validated in the total hip and total knee population. Additionally, the Patient Reported Outcomes Measurement Information System (PROMIS) Global-10 instrument, funded by the National Institute of Health, is increasingly used in the United States. PROMIS instruments use modern measurement theory to assess patient–reported health status for physical, mental, and social well–being to reliably and validly measure patient reported outcomes (PROs) for clinical research and practice. PROMIS instruments measure concepts such as pain, fatigue, physical function, depression, anxiety and social function. Hip disability and Osteoarthritis Outcome Score (HOOS) and Knee injury and Osteoarthritis Outcome Score (KOOS) are well validated and widely used instruments for measuring joint-specific pain and physical function before and after joint replacement. While the full HOOS and KOOS surveys are lengthy, the orthopedic community prefers an abbreviated survey that captures a subset of items referred to as HOOS JR/KOOS JR.
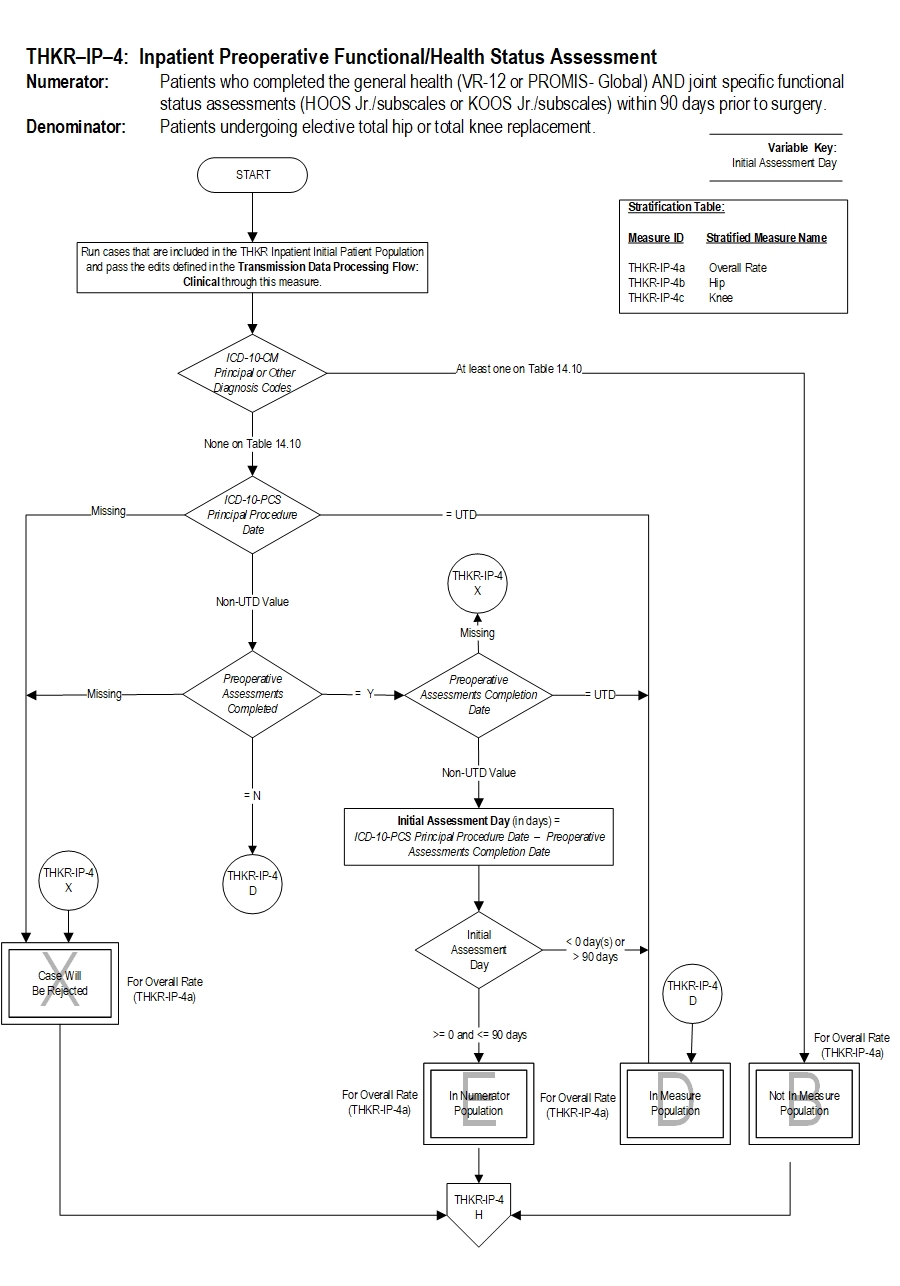
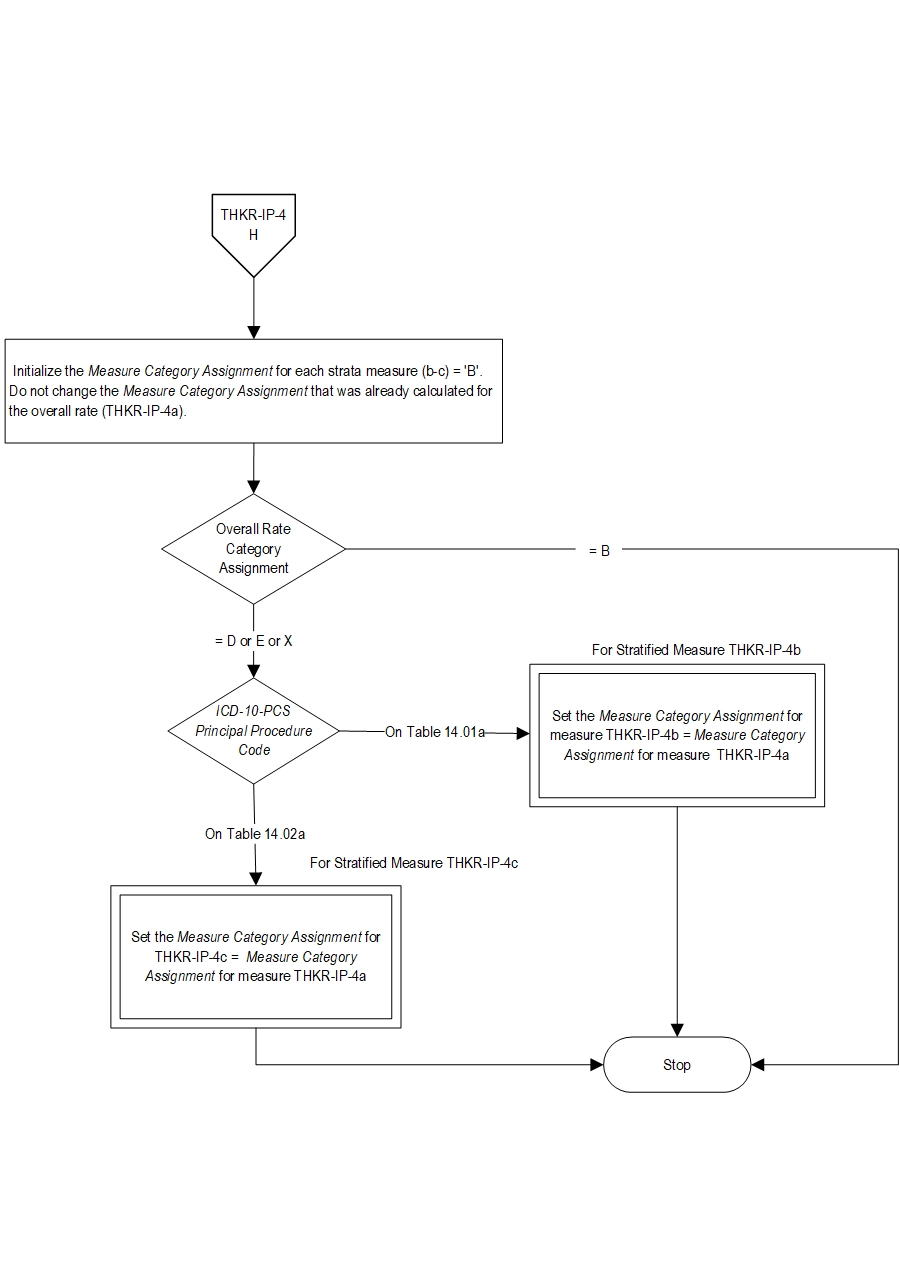
Type Of Measure: Process Improvement Noted As: Increase in the rateIncluded Populations: As above Excluded Populations: None Data Elements:Denominator Statement: Patients undergoing total hip or total knee replacement.
Included Populations:Excluded Populations:
- Patients with an ICD-10-PCS Principal Procedure Code for THKR as defined in Appendix A: Table 1a-Total Hip Replacements or Table-1b Total Knee Replacements
Data Elements:
- Patients less than 18 years of age
- Patients who have a Length of Stay greater than 120 day
- Patients with an ICD-10-PCS Other Procedure Code as defined in Appendix A: Table 14.05a (partial hip and partial knee replacements), Table 14.06a (revision and resurfacing procedures), Table 14.07a (removal of implanted devices/prostheses)
- Patients with an ICD-10-CM Principal Diagnosis Code or ICD-10-CM Other Diagnosis Code as defined in Appendix A: Table 14.08 (complication of internal fixation device/prosthesis), Table 14.09 (malignant neoplasm of the pelvis, sacrum, coccyx, lower limbs, or bone/bone marrow or a disseminated malignant neoplasm), or Table 14.10 (femur, hip, pelvic fracture)
- 1 Cella D, Hahn EA, Jensen SE, Patient-Reported Outcomes in Performance Measurement, ©2015 Research Triangle Institute.
- 2 Ayers DC, Zheng H, Franklin PD. Integrating Patient-reported Outcomes Into Orthopaedic Clinical Practice: Proof of Concept From FORCE-TJR. Clinical Orthopaedics and Related Research. 2013;471(11):3419-3425. doi:10.1007/s11999-013-3143-z.
- 3 Patient-Reported Outcomes Following Elective Primary Total Hip and/or Total Knee Arthroplasty: Hospital-Level Performance Measure(s), Phase 3 Measure Methodology Report, Yale New Haven Health Services Corporation – Center for Outcomes Research and Evaluation (CORE), May 2015.
- 4 AAOS letter to Andy Slavitt, Acting Administrator/CMS dated April 3, 2015. Re.: Response during public comment on “Proposed Electronic Clinical Quality Measures for Functional Status Assessment and Improvement for Patients who received a Total Hip Replacement and Functional Status Assessment and Improvement for Patients who received a Total Knee Replacement.”
- 5 AAHKS letter to Andy Slavitt, Acting Administrator/CMS dated March 30, 2015. Re.: Response during public comment on “Proposed Electronic Clinical Quality Measures for Functional Status Assessment and Improvement for Patients who received a Total Hip Replacement and Functional Status Assessment and Improvement for Patients who received a Total Knee Replacement.”
- 6 Patient Reported Outcomes Summit for Total Joint Arthroplasty Report, August 31, 2015.
- AAHKS sponsored Patient Reported Outcomes Summit for Total Joint Arthroplasty Report. August 31, 2015.
- FORCE-TJR letter to CMS dated September 3, 2015. Response during public comment on CMS-5516-Proposal for Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services.
- AAHKS, AAOS, AJRR letter to Andy Slavitt, Acting Administrator/CMS dated September 8, 2015. Response during public comment on CMS-5516-Proposal for Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services.
- AAHKS letter to Andy Slavitt, Acting Administrator/CMS dated September 8, 2015. Response during public comment on CMS-5516-Proposal for Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services.
- Hyperlinks to Patient-Reported Outcome Survey Instruments:
- HOOS http://www.koos.nu/
- HOOS Jr. https://www.hss.edu/hoos-jr-koos-jr-outcomes-surveys.asp
- KOOS http://www.koos.nu/
- KOOS Jr. https://www.hss.edu/hoos-jr-koos-jr-outcomes-surveys.asp
- VR-12 http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/
- PROMIS-Global http://www.nihpromis.org/measures/availableinstruments
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.