Measure Information Form
Version 2020A2
Measure Information Form
| Set Measure ID | Performance Measure Name |
|---|---|
| SUB-3 | Alcohol & Other Drug Use Disorder Treatment Provided or Offered at Discharge |
| SUB-3a | Alcohol & Other Drug Use Disorder Treatment at Discharge |
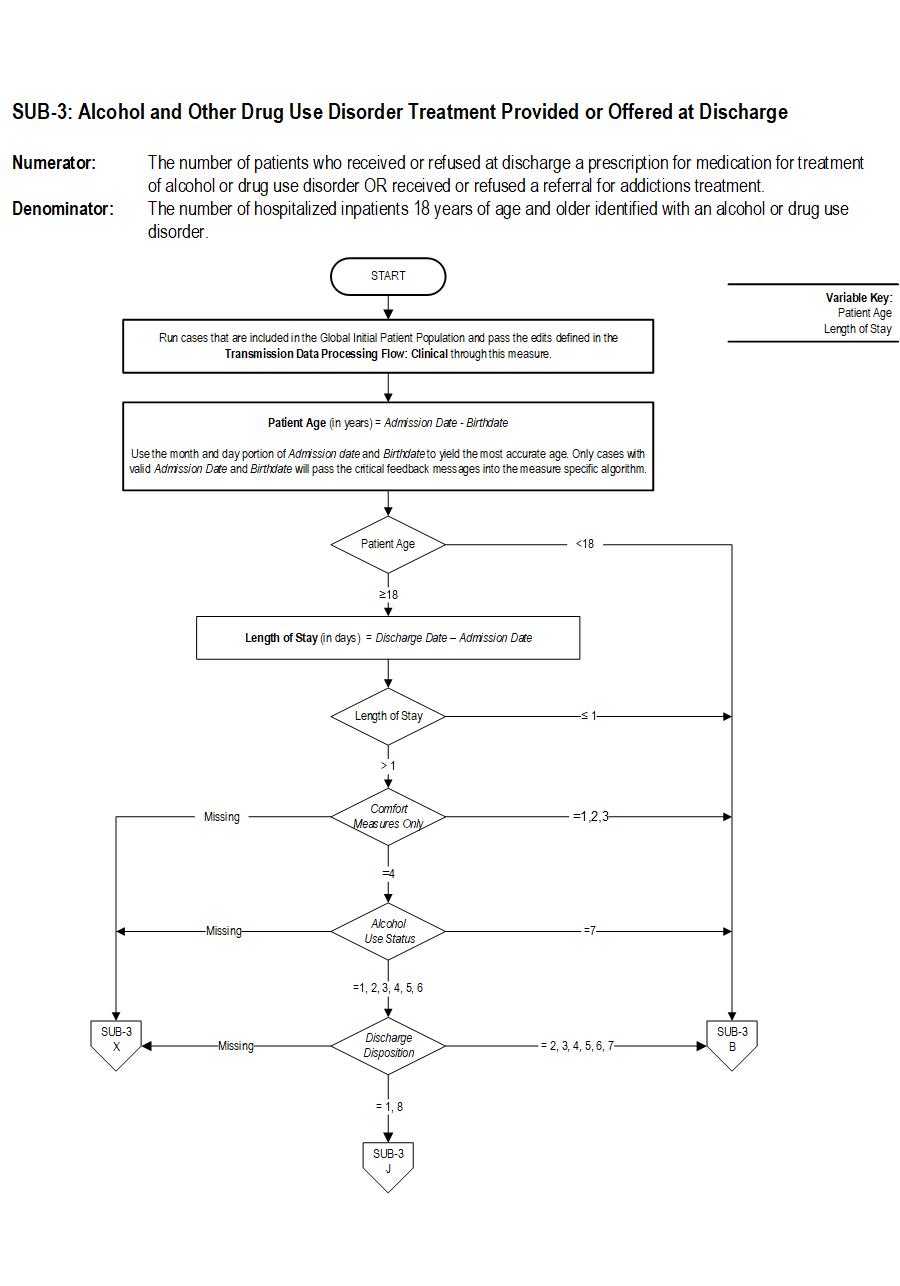
SUB-3 Patients who are identified with alcohol or drug use disorder who receive or refuse at discharge a prescription for FDA-approved medications for alcohol or drug use disorder, OR who receive or refuse a referral for addictions treatment.
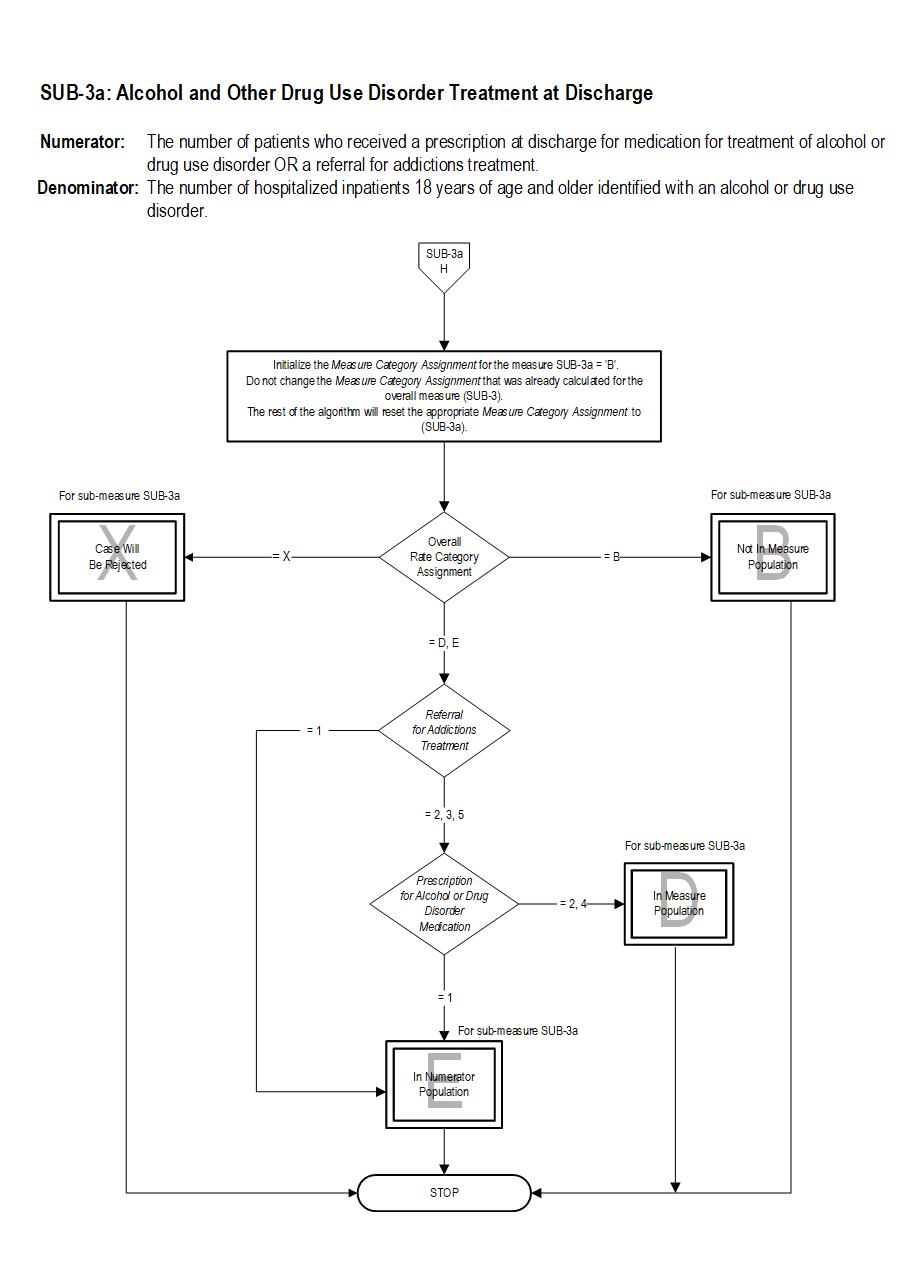
SUB-3a Patients who are identified with alcohol or drug disorder who receive a prescription for FDA-approved medications for alcohol or drug use disorder OR a referral for addictions treatment.
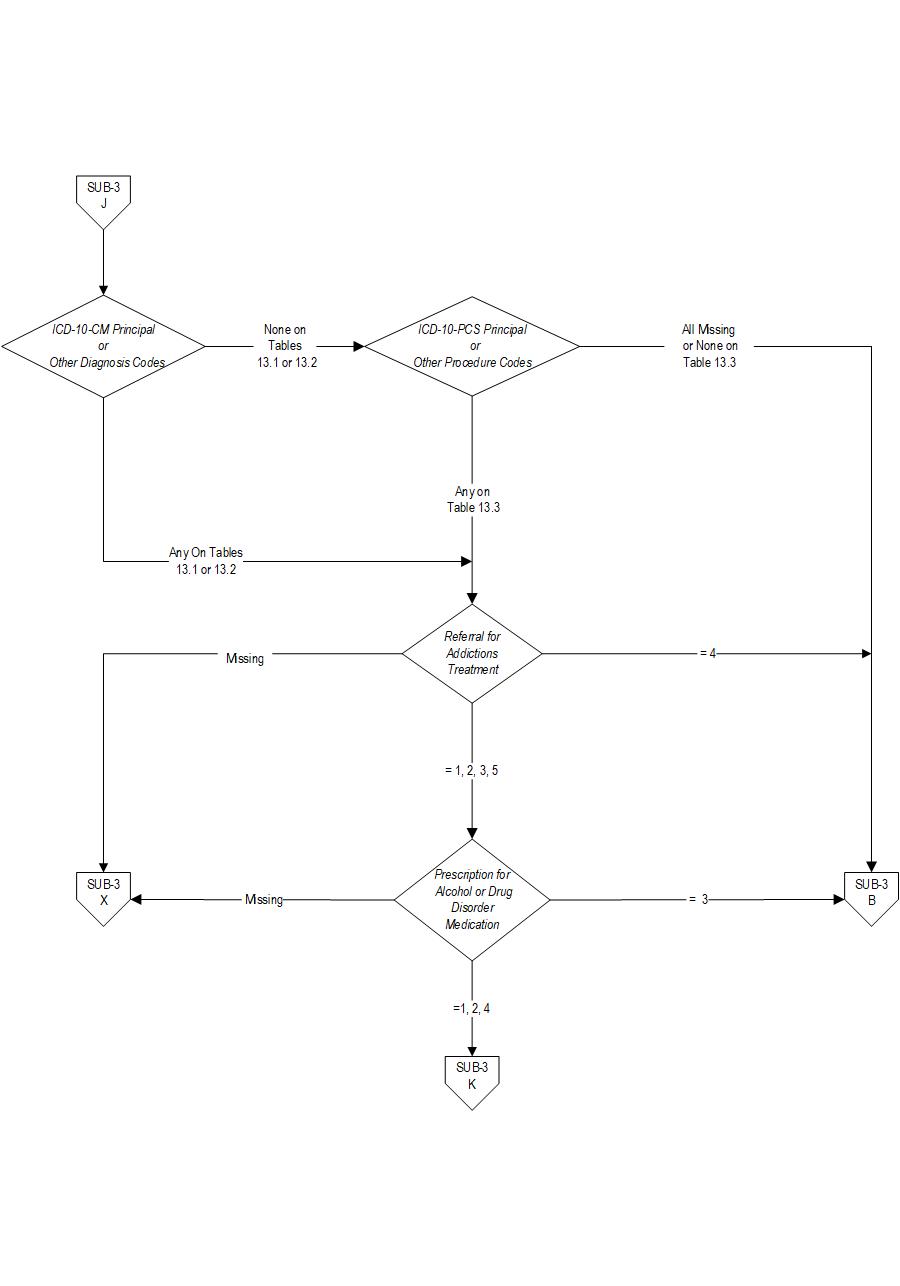
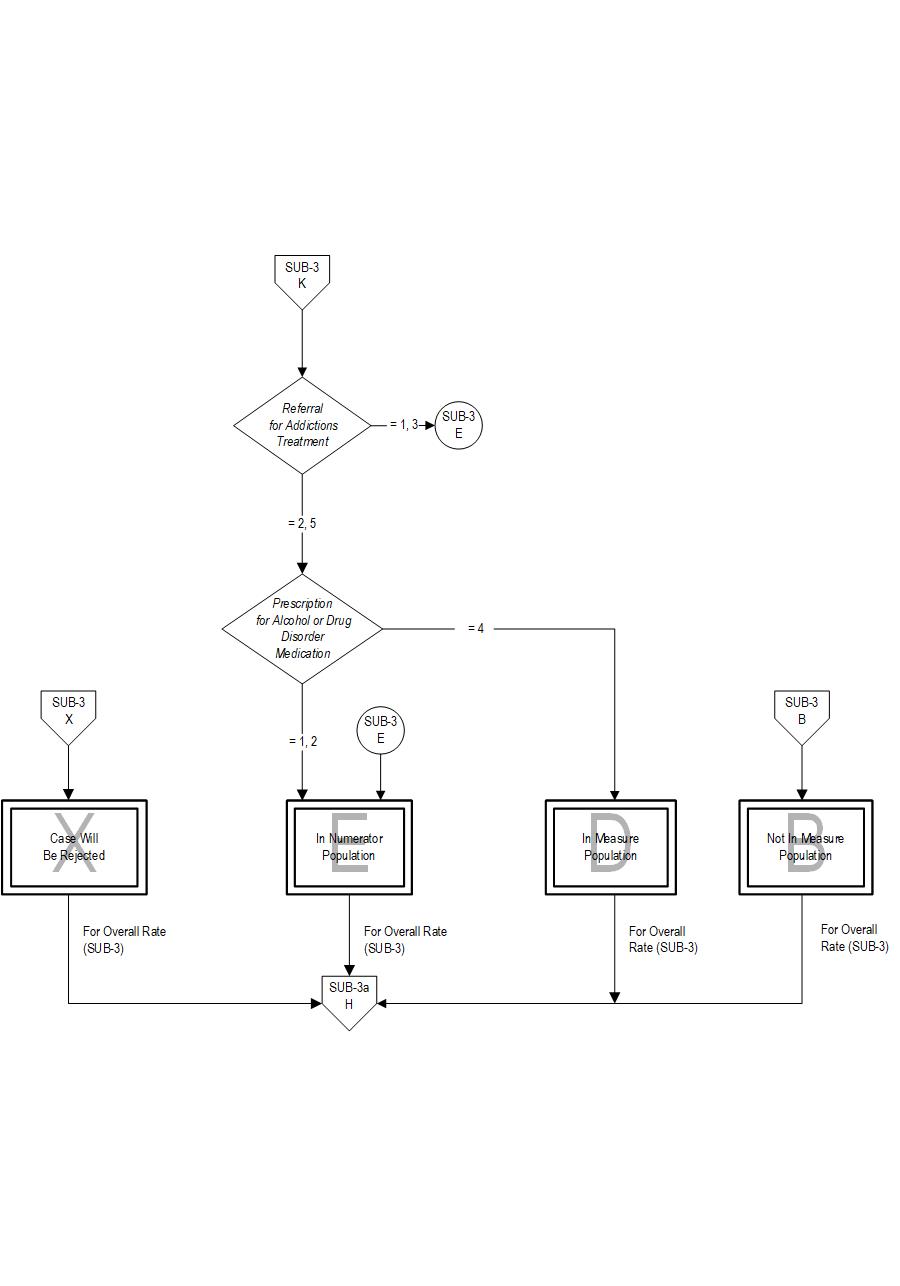
The measure is reported as an overall rate which includes all patients to whom alcohol or drug use disorder treatment was provided, or offered and refused, at the time of hospital discharge, and a second rate, a subset of the first, which includes only those patients who received alcohol or drug use disorder treatment at discharge. The Provided or Offered rate (SUB-3) describes patients who are identified with alcohol or drug use disorder who receive or refuse at discharge a prescription for FDA-approved medications for alcohol or drug use disorder, OR who receive or refuse a referral for addictions treatment. The Alcohol and Other Drug Disorder Treatment at Discharge (SUB-3a) rate describes only those who receive a prescription for FDA-approved medications for alcohol or drug use disorder OR a referral for addictions treatment. Those who refused are not included.
Rationale: Excessive use of alcohol and drugs has a substantial harmful impact on health and society in the United States. It is a drain on the economy and a source of enormous personal tragedy (The National Quality Forum, A Consensus Report 2007). In 1998 the economic costs to society were $185 billion dollars for alcohol misuse, and 143 billion dollars for drug misuse (Harwood 2000). Health care spending was 19 billion dollars for alcohol problems, and 14 billion dollars was spent treating drug problems.Nearly a quarter of a trillion dollars per year in lost productivity is attributable to substance use. More than 537,000 die each year as a consequence of alcohol, drug, and tobacco use making use of these substances the cause of one out of four deaths in the United States (Mokdad 2005).
An estimated 22.6 million adolescents and adults meet criteria for a substance use disorder. In a multi-state study that screened 459,599 patients in general hospital and medical settings, 23% of patients screened positive (Madras 2009).
Clinical trials have demonstrated that brief interventions, especially prior to the onset of addiction, significantly improve health and reduce costs, and that similar benefits occur in those with addictive disorders who are referred to treatment (Fleming 2002).
In a study on the provision of evidence-based care and preventive services provided in hospitals for 30 different medical conditions, quality varied substantially according to diagnosis. Adherence to recommended practices for treatment of substance use ranked last, with only 10% of patients receiving proper care (Gentilello 2005). Currently, less than one in twenty patients with an addiction are referred for treatment (Gentilello 1999).
Hospitalization provides a prime opportunity to address the entire spectrum of substance use problems within the health care system (Gentilello 2005, 1999). Approximately 8% of general hospital inpatients and 40 to 60 percent of traumatically-injured inpatients and psychiatric inpatients have substance use disorders (Gentilello 1999).
Type Of Measure: Process Improvement Noted As: Increase in the rateSUB-3: The number of patients who received or refused at discharge a prescription for medication for treatment of alcohol or drug use disorder OR received or refused a referral for addictions treatment. SUB-3a: The number of patients who received a prescription at discharge for medication for treatment of alcohol or drug use disorder OR a referral for addictions treatment.
Included Populations: Sub-3Denominator Statement: The number of hospitalized inpatients 18 years of age and older identified with an alcohol or drug use disorder.
Patients who refused a prescription for FDA-approved medication for treatment of an alcohol or drug dependence. Patients who refused a referral for addictions treatment. Sub-3a
Not Applicable Excluded Populations: SUB-3 and SUB-3a
None Data Elements:
Included Populations:Excluded Populations:
- Patients with ICD-10-CM Principal or Other Diagnosis Code for alcohol or drug use disorder listed on Table 13.1 and 13.2
- Patients with a Principal or Other ICD-10-PCS Procedure Code listed on Table 13.3
Data Elements:
- Patients less than 18 years of age
- Patient drinking at unhealthy levels who do not meet criteria for an alcohol use disorder
- Patients who are cognitively impaired
- Patients who expire
- Patients discharged to another hospital
- Patients who left against medical advice
- Patients discharged to another healthcare facility
- Patients discharged to home or another healthcare facility for hospice care
- Patients who have a duration of stay less than or equal to one day or greater than 120 days
- Patients who do not reside in the United States
- Patients receiving Comfort Measures Only documented
Variation may exist in the assignment of ICD-10 codes; therefore, coding practices may require evaluation to ensure consistency.
Measure Analysis Suggestions: Hospitals may wish to analyze data to show patients that refused both a medication prescription and referral and those who refused only one or the other. Sampling: Yes. Yes, please refer to the measure set specific sampling requirements and for additional information see the Population and Sampling Specifications section. Data Reported As: Aggregate rate generated from count data reported as a proportion. Selected References:- Bernstein J, Bernstein E, Tassiopoulos K, Heren T, Levenson S, Hingson R. Brief motivational interventions at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005 Jan 7;77(1):49-59.
- Fleming MF, Mundt MP, French MT, Manwell LB, Stauffacher EA, Barry KL. Brief physician advice for problem drinkers: Long-term efficacy and cost-benefit analysis. Alcohol Clin Exp Res. 2002 Jan;26(1):36-43.
- Gentilello LM, Ebel BE, Wickizer TM, Salkever DS Rivera FP. Alcohol interventions for trauma patients treated in emergency departments and hospitals: A cost benefit analysis. Ann Surg. 2005 Apr;241(4):541-50.
- Gentilello LM, Villaveces A, Ries RR, Nason KS, Daranciang E, Donovan DM Copass M, Jurkovich GJ Rivara FP. Detection of acute alcohol intoxication and chronic alcohol dependence by trauma center staff. J Trauma. 1999 Dec;47(6):1131-5; discussion 1135-9.
- Harwood, HJ, 2000. Updating Estimates of the Economic Costs of Alcohol Abuse in the United States. National Institute on Alcohol Abuse and Alcoholism. Available from: http://pubs.niaaa.nih.gov/publications/economic-2000/, Office of National Drug Control Policy. The Economic Costs of Drug Abuse in the United States: 1992–2002. Washington, DC: Executive Office of the President (Publication No. 207303), 2004.
- Havassy BE, Alvidrez J, Owen KK. Comparisons of patients with comorbid psychiatric and substance use disorders: implications for treatment and service delivery. Am J Psychiatry. 2004 Jan;161(1):139-45.
- Kirchner JE, Owen RR, Nordquist C, Fischer EP. Diagnosis and management of substance use disorders among inpatients with schizophrenia. Psychiatr Serv. 1998 Jan;49(1):82-5.
- Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: Comparison at intake and 6 months later. Drug Alcohol Depend. 2009 Jan 1;99(1-3):280-95. Epub 2008 Oct 16.
- Mokdad AH, Marks JS, Stroup DS, Gerberding JL. Actual Causes of Death in the United States, 2000. JAMA. 2004 Mar 10;291(10):1238-45 (Erratum in: JAMA. 2005 Jan 19;293(3):293-4.)
- McGlynn EA, Asch SM, Adams J. The Quality of Healthcare Delivered to Adults in the United States. N Engl J Med. 2003 Jun 26;348(26):2635-45.
- Prochaska JJ, Gill PH, Stephen E, Hall SM. Identification and Treatment of Substance Misuse on an Inpatient Psychiatry Unit. Psychiatr Serv. 2005 Mar;56(3):347-9.
- Smothers BA, Yahr HT, Ruhl CE. Detection of alcohol use disorders in general hospital admissions in the United States. Arch Intern Med. 2004 Apr 12;164(7):749-56.
- The National Quality Forum, National Voluntary Consensus Standards for the Treatment of Substance Use Conditions: Evidence-Based Treatment Practices; A Consensus Report; 2007.
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.