Measure Information Form
Version 2020A1
Measure Information Form
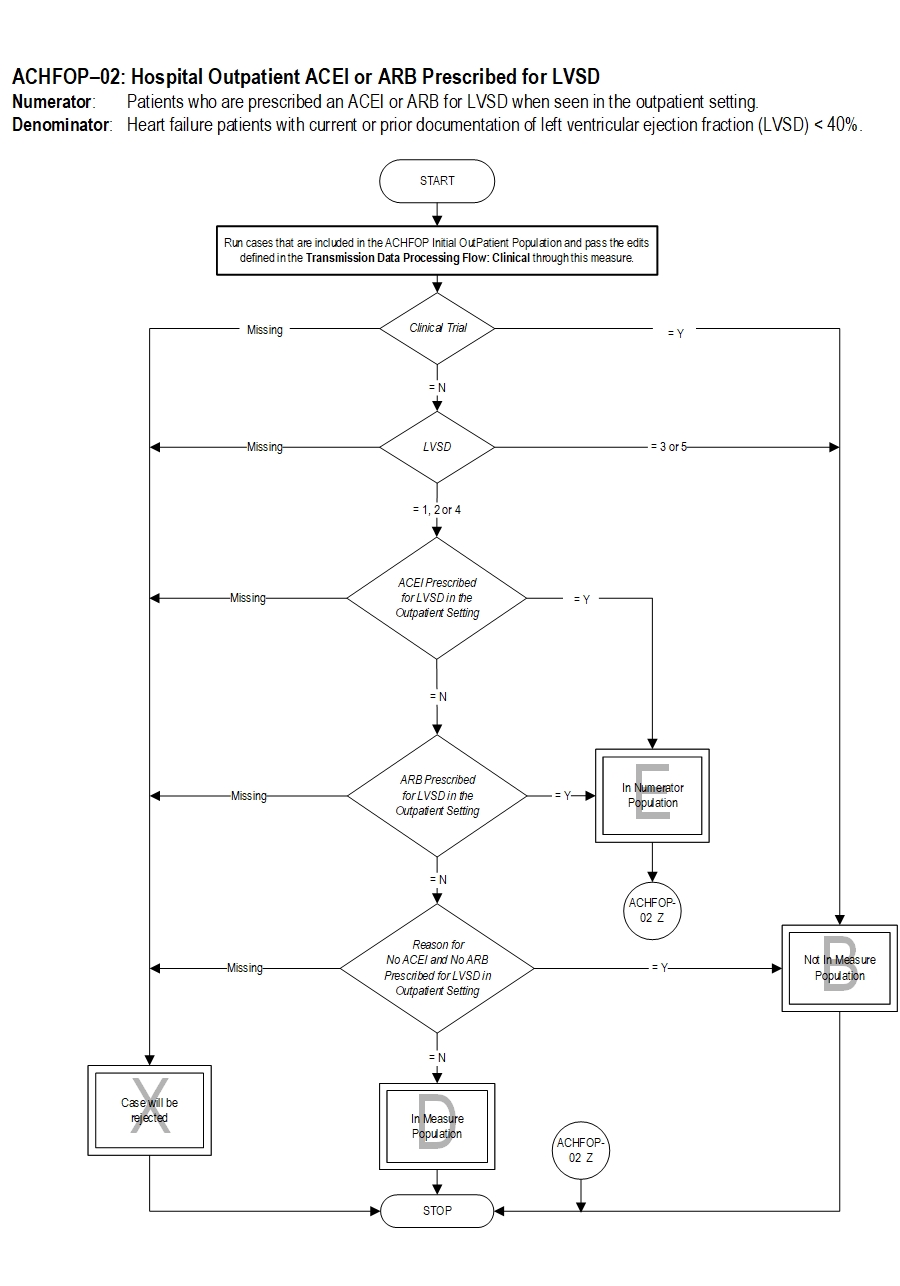
Included Populations: Not applicable Excluded Populations: None Data Elements:Denominator Statement: Heart failure patients with current or prior documentation of left ventricular ejection fraction (LVSD) < 40%.
Included Populations:Excluded Populations:
- E/M Code for hospital outpatient encounter as defined in Appendix A, Table 2.0
- An ICD-10-CM Principal Diagnosis Code for HF as defined in Appendix A, Table 2.1, and
- Documentation of LVSD < 40%
Data Elements:
- Patients renrolled in a Clinical Trial
- Patients who had a left ventricular assist device (LVAD) or heart transplant procedure (ICD-10-PCS Procedure Code for LVAD or heart transplant as defined in Appendix A, Table 2.2)
- Patients less than 18 years of age
- Patients with a documented Reason for No ACEI or ARB Prescribed for LVSD in the Outpatient Setting
- Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE, Goodlin SJ, et al. January 2011. American College of Cardiology Foundation/ American Heart Association/ Physician Consortium for Performance Improvement Heart Failure Performance Measurement Set. In American Medical Association. Retrieved February 2011, from http://www.ama-assn.org/ama1/pub/upload/mm/pcpi/hfset-12-5.pdf.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429-1435.
- Executive Council of the Heart Failure Society of America. Implications of recent clinical trials for heart failure performance measures. HFSA Position Statement. J Card Fail. 2004;10:4-5.
- Granger CB, McMurray JJ, Yusuf S et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772-776.
- Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al, writing on behalf of the 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult Writing Committee. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;53:1343— 82.
- Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, et al. Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail 2010; 16:475-539.
- Masoudi FA, Rathore SS, Wang Y et al. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110:724-731.
- Pfeffer MA, McMurray JJ, Velazquez EJ et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893-1906.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293-302.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327.
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.