Measure Information Form
Version 2020A
Measure Information Form
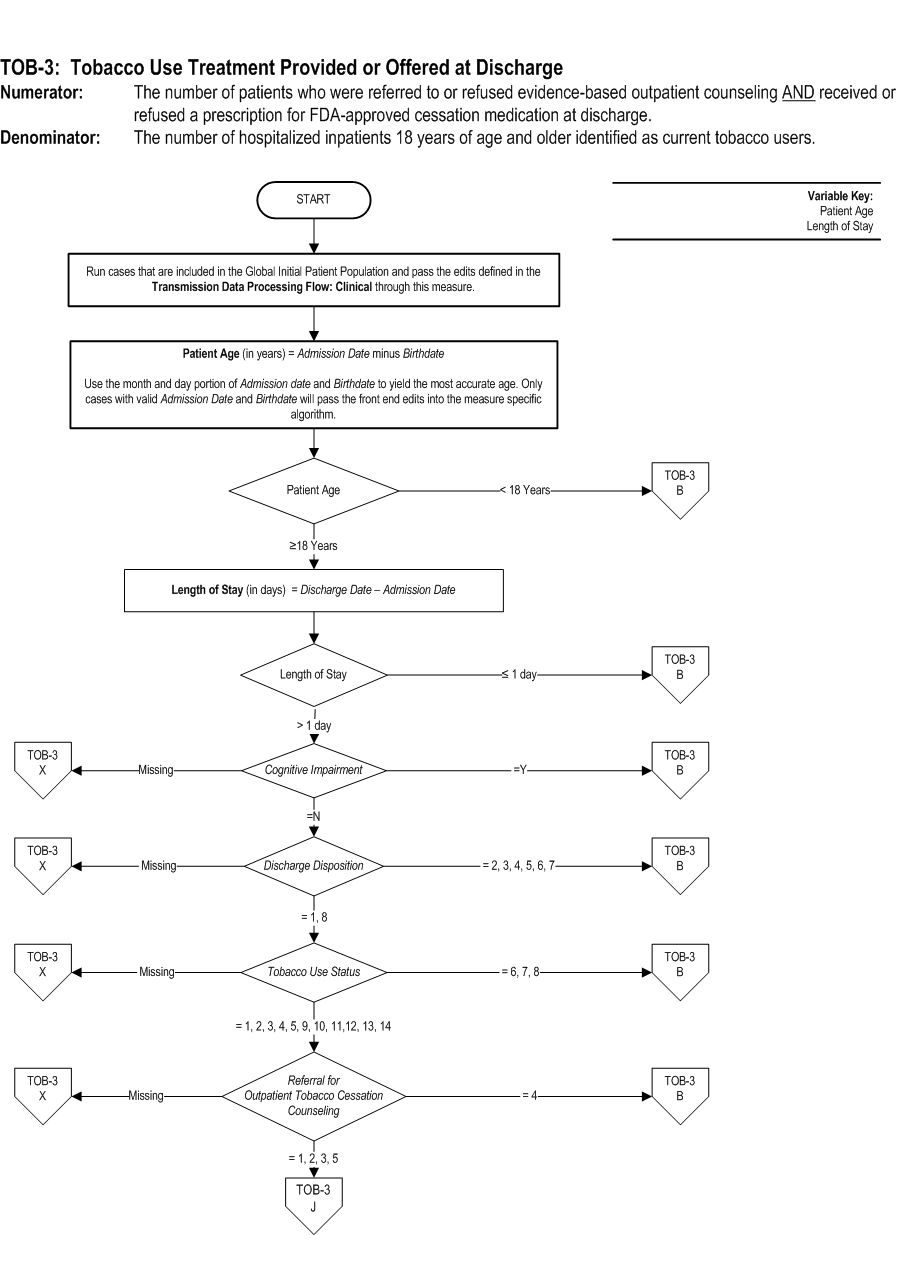
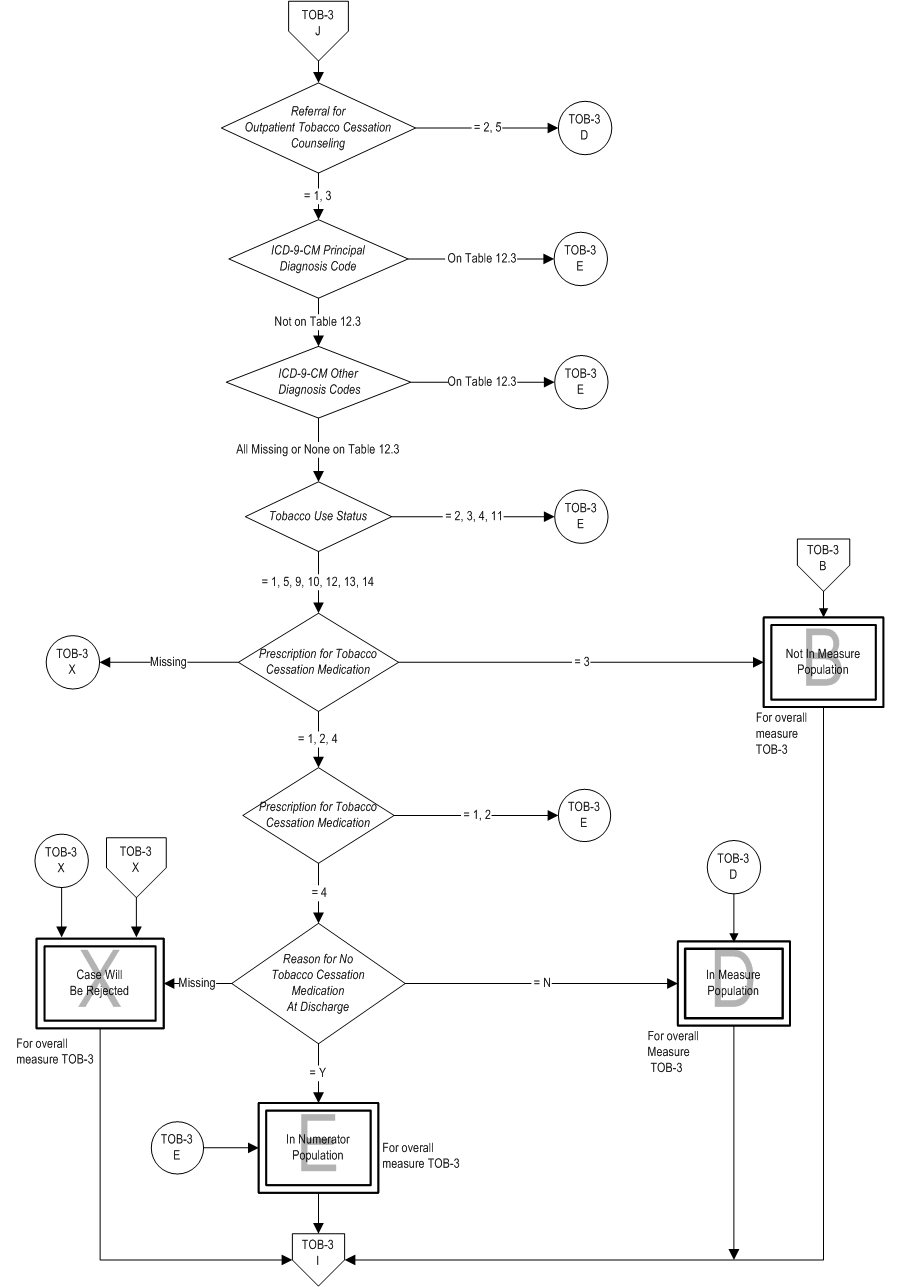
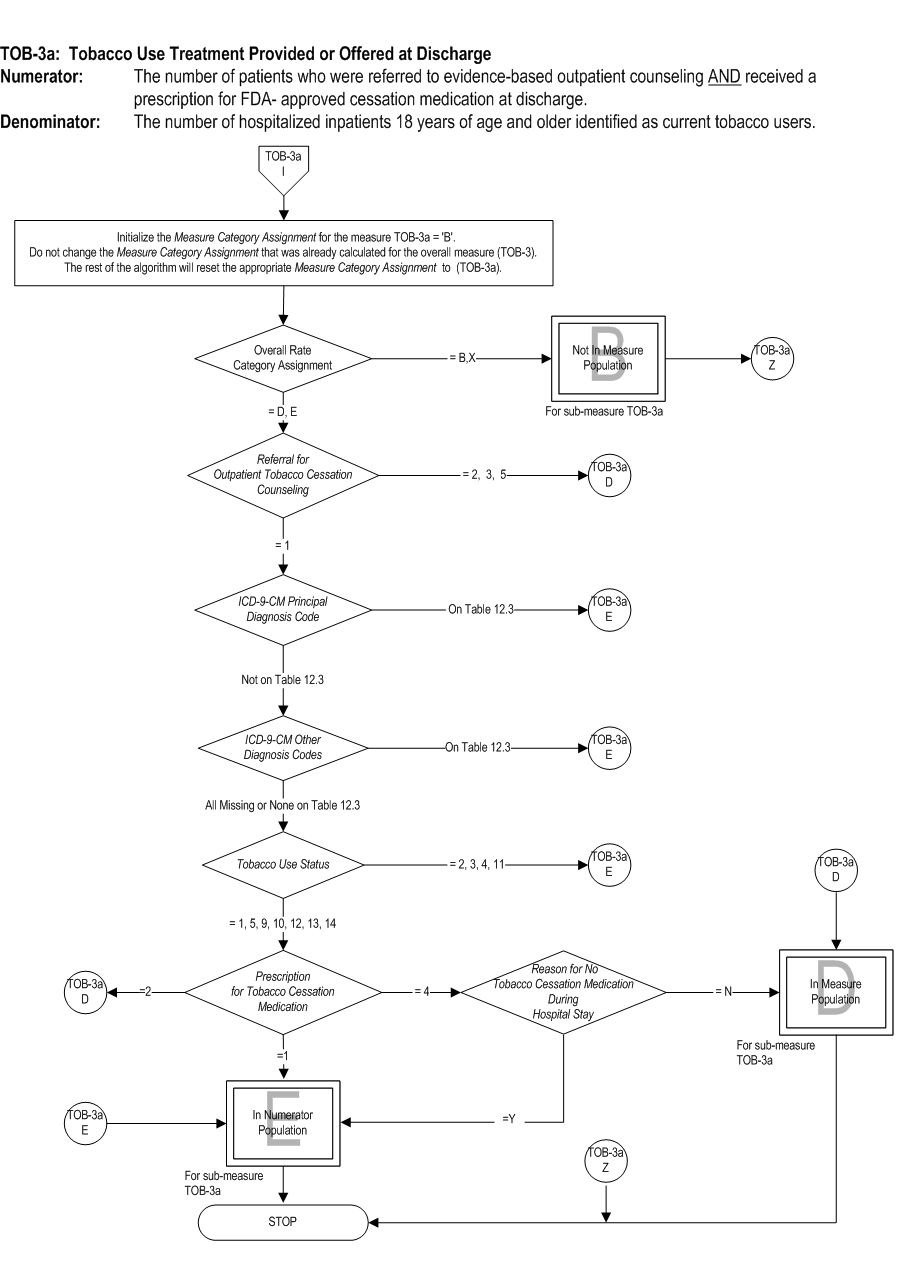
TOB-03: The number of patients who were referred to or refused evidence bassed outpatient counseling AND received or refused a prescription for FDA-approved cessation medication at discharge. TOB-03a: The number of patients who were referred to evidence-based outpatient counseling AND received a prescription for FDA-approved cessation medication at discharge. | * | *TOB-03 | TOB-03a | | Included Population | Patients who refused a prescription for FDA-approved tobacco cessation medication at discharge
Patients who refused a referral to evidence based outpatient counseling | Not Applicable | | Excluded Population | Smokeless tobacco users
Pregnant smokers
Light smokers
Patients with reasons for not administering FDA-approved cessation medication | Smokeless tobacco users
Pregnant smokers
Light smokers
Patients with reasons for not administering FDA-approved cessation medication |
Included Populations: Excluded Populations: Data Elements:Denominator Statement: The number of hospitalized inpatients 18 years of age and older identified as current tobacco users.
Included Populations: Not applicable Excluded Populations:Data Elements:
- Patients less than 18 years of age
- Patients who are cognitively impaired
- Patients who are not current tobacco users
- Patients who refused or were not screened for tobacco use status during the hospital stay
- Patients who have a duration of stay less than or equal to one day and greater than 120 days
- Patients who expired
- Patients who left against medical advice
- Patients discharged to another hospital
- Patients discharged to another health care facility
- Patients discharged to home for hospice care
- Patients who do not reside in the United States
Variation may exist in the assignment of ICD-9-CM codes; therefore, coding practices may require evaluation to ensure consistency.
Measure Analysis Suggestions: Hospitals may wish to identify those patients that refused either counseling or medications or both at discharge so as to have a better understanding of which type of treatment was accepted or refused so that efforts can be directed toward improving care. Sampling: Yes. For additional information see the Population and Sampling Specifications section Data Reported As: Aggregate rate generated from count data reported as a proportion. Selected References: 1. Centers for Disease Control and Prevention. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity LossesUnited States, 2000-2004. Morbidity and Mortality Weekly Report (MMWR) 2008. 57(45): 1226-1228. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a3.htm-/.2. McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA 1993 Nov 10;270(18):2207-12.
3. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2004.
4. Centers for Disease Control and Prevention. Best Practices for Comprehensive Tobacco Control Programs2007. Atlanta, GA, Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2007.
5. U.S. Department of Health and Human Services. Reducing tobacco use: a report of the Surgeon General. Atlanta, GA, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2000.
6. Baumeister SE, Schumann A, Meyer C, et al. Effects of smoking cessation on health care use: is elevated risk of hospitalization among former smokers attributable to smoking-related morbidity? Drug Alcohol Depend. 2007 May 11;88(2-3):197-203. Epub 2006 Nov 21.
7. Lightwood JM. The economics of smoking and cardiovascular disease. Prog Cardiovasc Dis. 2003 Jul-Aug;46(1):39-78.
8. Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997 Aug 19;96(4):1089-96.
9. Rasmussen SR, Prescott E, Sorensen TI, et al. The total lifetime health cost savings of smoking cessation to society. Eur J Public Health. 2005 Dec;15(6):601-6. Epub 2005 Jul 13.
10. Hurley SF. Short-term impact of smoking cessation on myocardial infarction and stroke hospitalizations and costs in Australia. Med J Aust. 2005 Jul 4;183(1):13-7.
11. Critchley J, Capewell S. Smoking cessation for the secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2004;(1):CD003041.
12. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med. 2007 Jun 7;356(23):2388-98.
13. Fiore MC et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service. May 2008.
14. U.S. Department of Health and Human Services: The health benefits of smoking cessation: a report of the Surgeon General. Publication No. (CDC) 90-8416. Rockville, MD: U.S. Department of Health and Human Services, 1990.
15. Rigotti NA, Munafo MR, Stead LF. Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med. 2008 Oct 13;168(18):1950-60.
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.