Measure Information Form
Version 2019A1
Measure Information Form
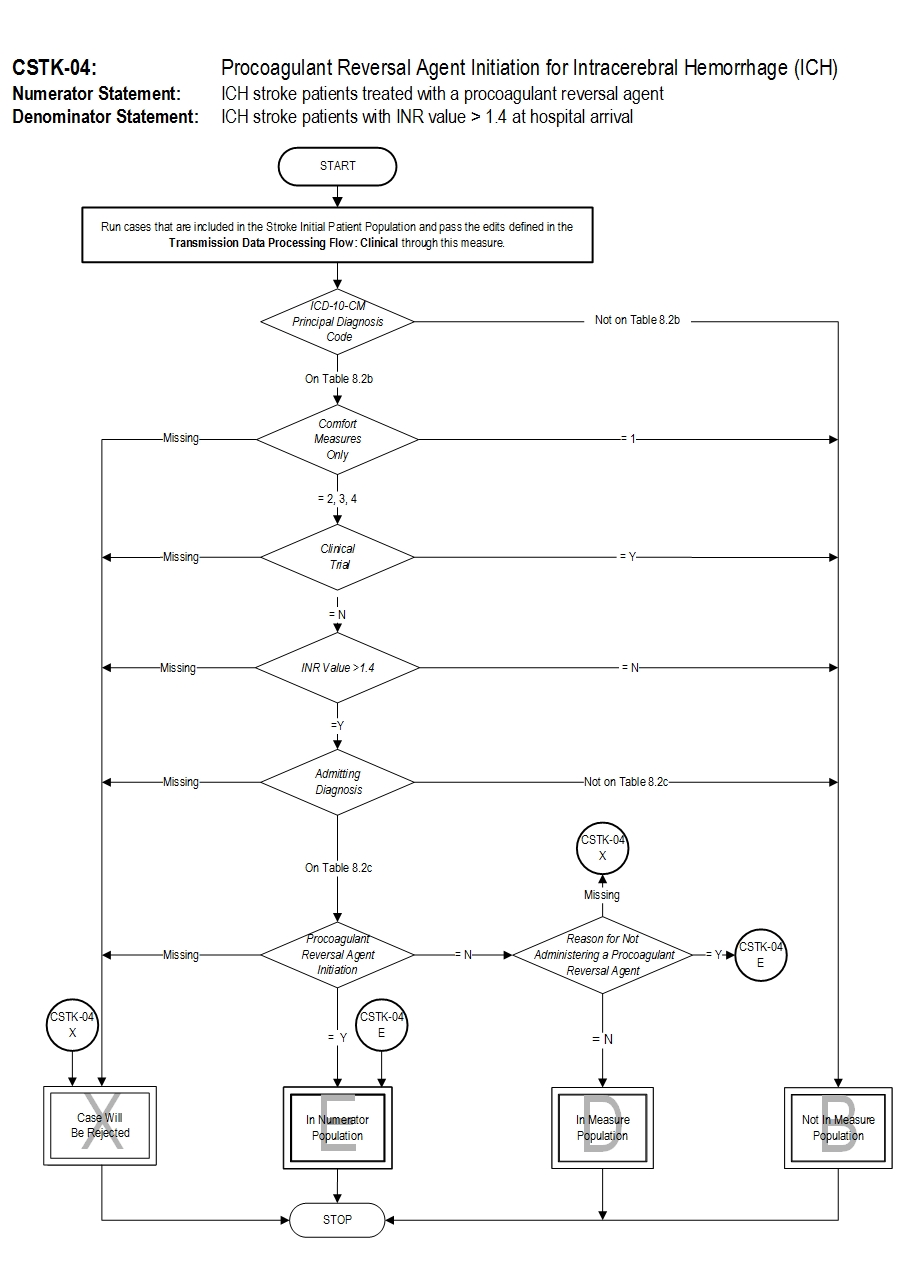
Included Populations: As above Excluded Populations: None Data Elements:Denominator Statement: ICH stroke patients with INR value > 1.4 at hospital arrival.
Included Populations:Risk Adjustment: No. Data Collection Approach: Retrospective data sources for required data elements include administrative data and medical records. Data Accuracy: Variation may exist in the assignment of ICD-10 codes; therefore, coding practices may require evaluation to ensure consistency. Measure Analysis Suggestions: None Sampling: Yes. Please refer to the measure set specific sampling requirements and for additional information see the Population and Sampling Specifications section. Data Reported As: Aggregate rate generated from count data reported as a proportion. Selected References: 1. Ansell J, Hirsch J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidleines (8th Edition). Chest. 2008;133(suppl):160S-198S. 2. Fredriksson K, Norrving B, Strömblad, LG. Emergency reversal of anticoagulation after intracerebral hemorrhage. Stroke. 1992;23:972-977. 3. Frontera JA, Lewin JJ 3rd, Rabinstein AA, Aisiku IP, Alexandrov AW, Cook AM, del Zoppo GJ, Kumar MA, Peerschke EI, Stiefel MF, Teitelbaum JS, Wartenberg KE, Zerfoss CL. Guideline for reversal of antithrombotics in intracranial hemorrhage: a statement for healthcare professionals from the Neurocritical Care Society and Society of Critical Care Medicine. Neurocrit Care. 2016;24(1):6-46. 4. Goldstein JN, Thomas SH, Frontiero V, Joseph A, Engel C, Snider R, Smith EE, Greenberg SM, Rosand J. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151-155. 5. Hanley JP. Warfarin reversal. J Clin Pathol. 2004;57:1132-1139. 6. Hemphill JC III, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald L, Mitchell PH, Scott PA, Selim MH, Woo D. Guidelines for the management of spontaneous intracerebral hemorrhage:a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:1-29. 7. Leifer D, Bravata DM, Connors JJ III, Hinchey JA, Jauch EC, Johnston SC, Latchaw R, Likosky W, Ogilvy C, Qureshi AI, Summers D, Sung GY, Williams LS, Zorowitz R, on behalf of the American Heart Association Special Writing Group of the Stroke Council, Atherosclerotic Peripheral Vascular Disease Working Group and Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular Nursing. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow-up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:865-866. 8. Leissinger CA, Blatt PM, Hoots WK, Ewenstein B. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83:137-143. 9. Morgenstern LB, Hemphill JC III, Anderson C, Becker K, Broderick JP, Connolly ES Jr, Greenberg SM, Huang JN, Macdonald RL, Messé SR, Mitchell PH, Selim M, Tamargo RJ; and on behalf of the American Heart Association Stroke council and Council on Cardiovascular Nursing. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2111-2114. 10. Nilsson OG, Lindgren A, Ståhl N, Brandt L, Säveland H. Incidence of intracerebral and subarachnoid hemorrhage in southern Sweden. J Neurol Neurosurg Psychiatry. 2000;69:601-607. 11. Pabinger I, Brenner B, Kalina U, Knaub S, Nagy A, Ostermann H; Beriplex P/N Reversal Study Group. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622-631. 12. Rådberg JA, Olsson JE, Rådberg CT. Prognostic parameters in spontaneous intracerebral hematomas with special reference to anticoagulant treatment. Stroke. 1991;22:571-576. 13. Reiss H, Meier-Hellman A, Motsch J, Elias M, Kursten FW, Dempfle CE. Prothrombin complex concentrate (Octaplex) in patients requiring immediate reversal of oral anticoagulation. Thromb Res. 2007;121:9-16. 14. Rosovsky RP, Crowther, MA. What is the evidence for the off-label use of recombinant factor VII (rFVIIa) in the acute reversal of warfarin? Hematology Am Soc Hematol Educ Program. 2008:36-38. 15. Sjöblom L, Hårdemark HG, Lindgren A, Norrving B, Fahlén M, Samuelsson M, Stigendal L, Stockelberg D, Taghavi A, Wallrup L, Wallvik J. Mangement and prognostic features of intracerebral hemorrhage during anticoagulant therapy: a Swedish multicenter study. Stroke. 2001;32:2567-2574. 16. Steiner T, Kaste M, Katse M, Forsting M, Mendelow D, Kwiecinski H, Szikora I, Juvela S, Marchel A, Chapot R, Cognard C, Unterberg A. Hacke W. Recommendations for the management of intracranial haemorrhage - part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovascular Diseases. 2006;22(4):294-316. 17. Watson HG, Baglin T, Laidlaw SL, Makris M, Preston FE. A comparison of the efficacy and rate of response to oral and intravenous vitamin K in reversal of over-anticoagulation with warfarin. Haematol. 2001;115:145-149.Excluded Populations:
- Discharges with ICD-10-CM Principal Diagnosis Code for hemorrhagic stroke as defined in Appendix A, Table 8.2b for ICD-10 codes,
AND
- Patients who have an Admitting Diagnosis of primary parenchymal ICH,
AND
- INR >1.4 performed closest to hospital arrival
Data Elements:
- Patients less than 18 years of age
- Patients who have a Length of Stay > 120 days
- Patients with Comfort Measures Only documented on day of or after hospital arrival
- Patients enrolled in clinical trials
CPT® only copyright 2019 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association.
You, your employees and agents are authorized to use CPT® only as contained in The Joint Commission performance measures solely for your own personal use in directly participating in healthcare programs administered by The Joint Commission. You acknowledge that the American Medical Association (“AMA”) holds all copyright, trademark and other rights in CPT®.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT® for resale and/or license, transferring copies of CPT® to any party not bound by this Agreement, creating any modified or derivative work of CPT®, or making any commercial use of CPT®. License to use CPT® for any use not authorized herein must be obtained through the American Medical Association, Intellectual Property Services, AMA Plaza, 330 North Wabash Avenue, Suite 39300, Chicago, Illinois 60611-5885. Applications are available at the American Medical Association Web site, www.ama- assn.org/go/cpt.
U.S. Government Rights This product includes CPT® which is commercial technical data, which was developed exclusively at private expense by the American Medical Association, 330 North Wabash Avenue, Chicago, Illinois 60611. The American Medical Association does not agree to license CPT® to the Federal Government based on the license in FAR 52.227-14 (Data Rights - General) and DFARS 252.227-7015 (Technical Data - Commercial Items) or any other license provision. The American Medical Association reserves all rights to approve any license with any Federal agency.
Disclaimer of Warranties and Liabilities. CPT® is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to the implied warranties of merchantability and fitness for a particular purpose. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT®, and the (AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this product is with The Joint Commission, and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this product.
This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled “accept”.