Release Notes:
Measure Information Form
Version 2018A
Measure Information Form
Version 2018A
Measure Information Form
Hyperkalemia is a major risk of aldosterone antagonist therapy. Potassium supplements should be discontinued after the initiation of therapy, and patients should be counseled to avoid high-potassium foods.
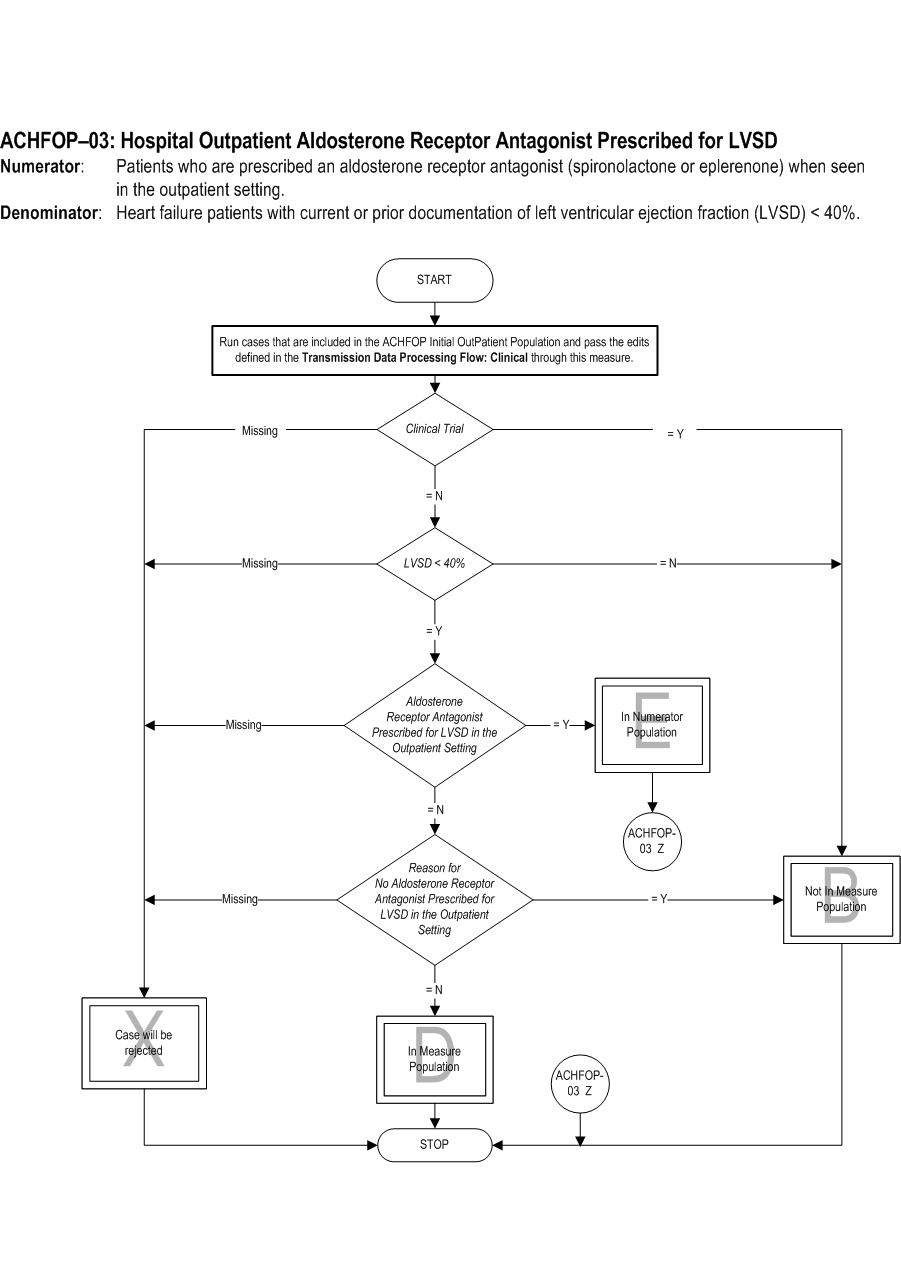
Type of Measure: Process Improvement Noted As: Increase in the rate Numerator Statement: Patients who are prescribed an aldosterone receptor antagonist (spironolactone or eplerenone) when seen in the outpatient setting
Risk Adjustment: No.
Data Collection Approach: Retrospective data sources for required data elements include administrative data and medical records.
Data Accuracy: Variation may exist in the assignment of ICD-10 codes; therefore, coding practices may require evaluation to ensure consistency.
Measure Analysis Suggestions: None.
Sampling: Yes. Please refer to the measure set specific sampling requirements and for additional information see the Population and Sampling Specifications section.
Data Reported As: Aggregate rate generated from count data reported as a proportion.
Selected References:
Included Populations: Not applicable Excluded Populations: None Data Elements:Denominator Statement: Heart failure patients with current or prior documentation of left ventricular ejection fraction (LVSD) < 40%.
Included Populations:Excluded Populations:
- E/M Code for hospital outpatient encounter as defined in Appendix A, Table 2.0
- An ICD-10-CM Principal Diagnosis Code for HF as defined in Appendix A, Table 2.1, and
- Documentation of LVSD < 40%
Data Elements:
- Clinical Trial
- Patients who had a left ventiricular assist device (LVAD) or heart transplant procedure (ICD-10-PCS Procedure Code for LVAD or heart transplant as defined in Appendix A, Table 2.2)
- Patients less than 18 years of age
- Patients with a documented Reason for No Aldosterone Receptor Antagonist Prescribed for LVSD in the Outpatient Setting
- Birthdate
- Clinical Trial
- Discharge Code
- E/M Code
- ICD-10-CM Principal Diagnosis Code
- ICD-10-PCS Other Procedure Codes
- ICD-10-PCS Principal Procedure Code
- ICD-10-PCS Principal Procedure Date
- LVSD < 40%
- Outpatient Encounter Date
- Reason for No Aldosterone Receptor Antagonist Prescribed for LVSD in the Outpatient Setting
- American Heart Association. Get With The Guidelines® Outpatient Fact Sheet. 2010.
- Hunt SA, Abraham WT, Chin MH, Felman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update incorporated Into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391-e479.
- Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Klapholz M, Applications/LocalApps.MoserDK, Rogers JG, Starling RC, Stevenson WG, Tang WHW, Teerlink JR, Walsh MN. Executive Summary: HFSA 2010 Comphrensive Heart Failure Practice Guideline. J Card Fail 2010;16:475-539.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, Applications/LocalApps.McBride PE, Applications/LocalApps.McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327.
| Related Topics |
Questions? Ask Question to Joint Commission staff
Copyright © 2018 by The Joint Commission.