Release Notes:
Measure Information Form
Version 2016B1
Measure Information Form
Measure Set: Comprehensive Stroke(CSTK)
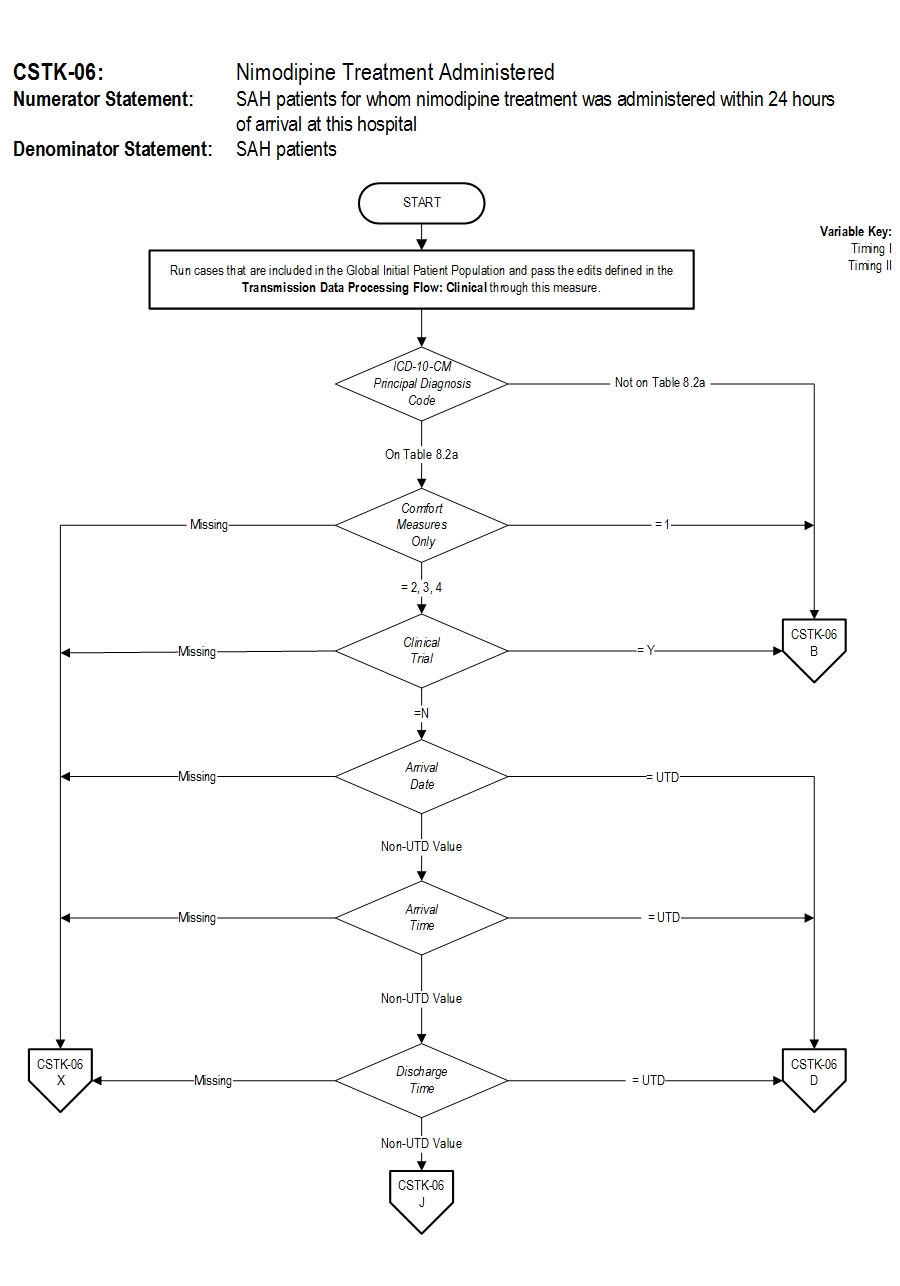
Set Measure ID: CSTK-06
Performance Measure Name: Nimodipine Treatment Administered
Description: Subarachnoid hemorrhage (SAH) patients for whom nimodipine treatment was administered within 24 hours of arrival at this hospital
Rationale: Cerebral vasopasm is a serious complication following SAH, occurring in 30% to 70% of patients and accounting for nearly 50% of the deaths in patients surviving to treatment. Constriction of the arterial lumen results in diminished cerebral perfusion distal to the affected artery, which produces a delayed neurological deficit that may progress to cerebral infarction without early management of the ruptured aneurysm. The arterial narrowing that occurs in cerebral vasospasm is typically a transient or temporary event, lasting from a few days up to 3 weeks. Oral nimodipine is a proven and valuable treatment to prevent
or limit the severity of cerebral vasospasm.
Type of Measure: Process
Improvement Noted As: Increase in the rate
Numerator Statement: SAH patients for whom nimodipine treatment was administered within 24 hours of arrival at this hospital.
Included Populations:
As above
Excluded Populations: None
Data Elements:
Denominator Statement: SAH patients
Included Populations:
Discharges with ICD-10-CM Principal Diagnosis Code for subarachnoid hemorrhage as defined in Appendix A, Table 8.2a for ICD-10 codes.
Excluded Populations:
- Patients less than 18 years of age
- Patients who have a Length of Stay > 120 days
- Patients with Comfort Measures Only documented on day of or after hospital arrival
- Patients enrolled in clinical trials
- Patients discharged within 24 hours of arrival at this hospital
Data Elements:
Risk Adjustment: No.
Data Collection Approach: Retrospective data sources for required data elements include administrative data and medical records.
Data Accuracy: Variation may exist in the assignment of ICD-10 codes; therefore, coding practices may require evaluation to ensure consistency.
Measure Analysis Suggestions: None
Sampling: Yes. Please refer to the measure set specific sampling requirements and for additional information see the Population and Sampling Specifications section.
Data Reported As: Aggregate rate generated from count data reported as a proportion.
Selected References:
1. Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks E. Guidelines for the Early Management of Adults with Ischemic Stroke: A Guideline From the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Stroke. 2007;38:1686.
2. Allen GS, Ahn HS, Presiosi TJ, Battye R,Boone SC, Cho SN, Kelly DL, Weir BK, Crabbe RA, Lavik PJ, Rosenbloom SB, Dorsey FC, Ingram CR, Mellits DE, Bertsch LA, Boisvert DP, Hundley MB, Johnson RK, Strom JA, Transou CR. Cerebral arterial spasm: a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308:619-624.
3. Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE, Patel AB, and Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 2009;40:1008-1011.
4. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guidelines for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1-27.
5. Fogelholm R, Palomaki H, Erila T, Rissanen A, Kaste M. Blood pressure, nimodipine, and outcome of ischemic stroke. Acta Neurol Scand. 2004;109:200-204.
6. Haley EC Jr, Kassell NF, Torner JC, Truskowski LL, Germanson TP. A randomized trial of two doses of nicardipine in aneurysmal subarachnoid hemorrhage: a report of the Cooperative Aneurysm Study. J Neurosugr. 1994;80:788-796.
7. Kaste M, Fogelholm R, Erila T, Palomaki H, Murros K, Rissanen A, Sarna S. A randomized, double-blinded, placebo-controlled trial of nimodipine in acute ischemic hemispheric stroke. Stroke. 1994;25:1348-1353.
8. Leifer D, Bravata DM, Connors JJ III, Hinchey JA, Jauch EC, Johnston SC, Latchaw R, Likosky W, Ogilvy C, Qureshi AI, Summers D, Sung GY, Williams LS, Zorowitz R, on behalf of the American Heart Association Special Writing Group of the Stroke Council, Atherosclerotic Peripheral Vascular Disease Working Group and Council on Cardiovascular Surgery and Anesthesia, and Council on Cardiovascular Nursing. Metrics for measuring quality of care in comprehensive stroke centers: detailed follow-up to Brain Attack Coalition comprehensive stroke center recommendations: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:863-864.
9. Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, Sternau LL, Torner J, Adams HP Jr, Feinberg W. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 1994;25:2315-2328.
10. Toyota BD. The efficacy of an abbreviated course of nimodipine in patients with good grade aneurysmal subarachnoid hemorrhage. JNeurosurg. 1999;90(2):203-206.
11. Wahlgren NG, Applications/LocalApps.MacMahon DG, Applications/LocalApps.DeKeyser J, Indredavik B, Ryman T. Intravenous Nimodipine West European Stroke Trial (INWEST) of nimodipine in the treatment of acute ischemic stroke. Cerebrovasc Dis. 1994;4:204-210.
12. The American Nimodipine Study Group. Clinical trial of nimodipine in acute ischemic stroke. Stroke. 1992;23:3-8.
Measure Algorithm: