Release Notes:
Measure Information Form
Version 2015A
Pneumonia (PN)
Set Measures
| Set Measure ID |
Measure Short Name |
| PN-3a |
Blood Cultures Performed Within 24 Hours Prior to or 24 Hours After Hospital Arrival for Patients Who Were Transferred or Admitted to the ICU Within 24 Hours of Hospital Arrival |
| PN-6 |
Initial Antibiotic Selection for Community-Acquired Pneumonia (CAP in Immunocompetent Patients) |
General Data Elements
| Element Name |
Collected For |
| Admission Date |
All Records, |
| Birthdate |
All Records, |
| CMS Certification Number |
Hospital Clinical Data File, Optional for All Records, |
| Discharge Date |
All Records, Not collected for HBIPS-2 and HBIPS-3 |
| Health Care Organization Identifier |
All Records, Aggregate Data File, Patient Population Data File, Hospital Clinical Data File, |
| Hispanic Ethnicity |
All Records, |
| ICD-9-CM Other Diagnosis Codes |
All Records, Optional for HBIPS-2, HBIPS-3 |
| ICD-9-CM Other Procedure Codes |
All Records, Optional for All HBIPS Records |
| ICD-9-CM Other Procedure Dates |
All Records, Optional for All HBIPS and PBM Records |
| ICD-9-CM Principal Diagnosis Code |
All Records, Optional for HBIPS-2 and HBIPS-3 |
| ICD-9-CM Principal Procedure Code |
All Records, Optional for All HBIPS Records |
| ICD-9-CM Principal Procedure Date |
All Records, Optional for All HBIPS and PBM Records |
| Payment Source |
All Records, Optional for HBIPS-2 and HBIPS-3 |
| Race |
All Records, |
| Sex |
All Records, |
Algorithm Output Data Elements
Measure Set Specific Data Elements
| Element Name |
Collected For |
| Arrival Date |
PN-3a, |
| Arrival Time |
PN-3a, |
| Blood Culture Collected |
PN-3a, |
| Chest X-Ray |
PN-3a, |
| Clinical Trial |
PN-3a, |
| Comfort Measures Only |
PN-3a, |
| ICU Admission or Transfer |
PN-3a, |
| Initial Blood Culture Collection Date |
PN-3a, |
| Initial Blood Culture Collection Time |
PN-3a, |
| Pneumonia Diagnosis: ED/Direct Admit |
PN-3a, |
| Transfer From Another Hospital or ASC |
PN-3a, |
Related Materials
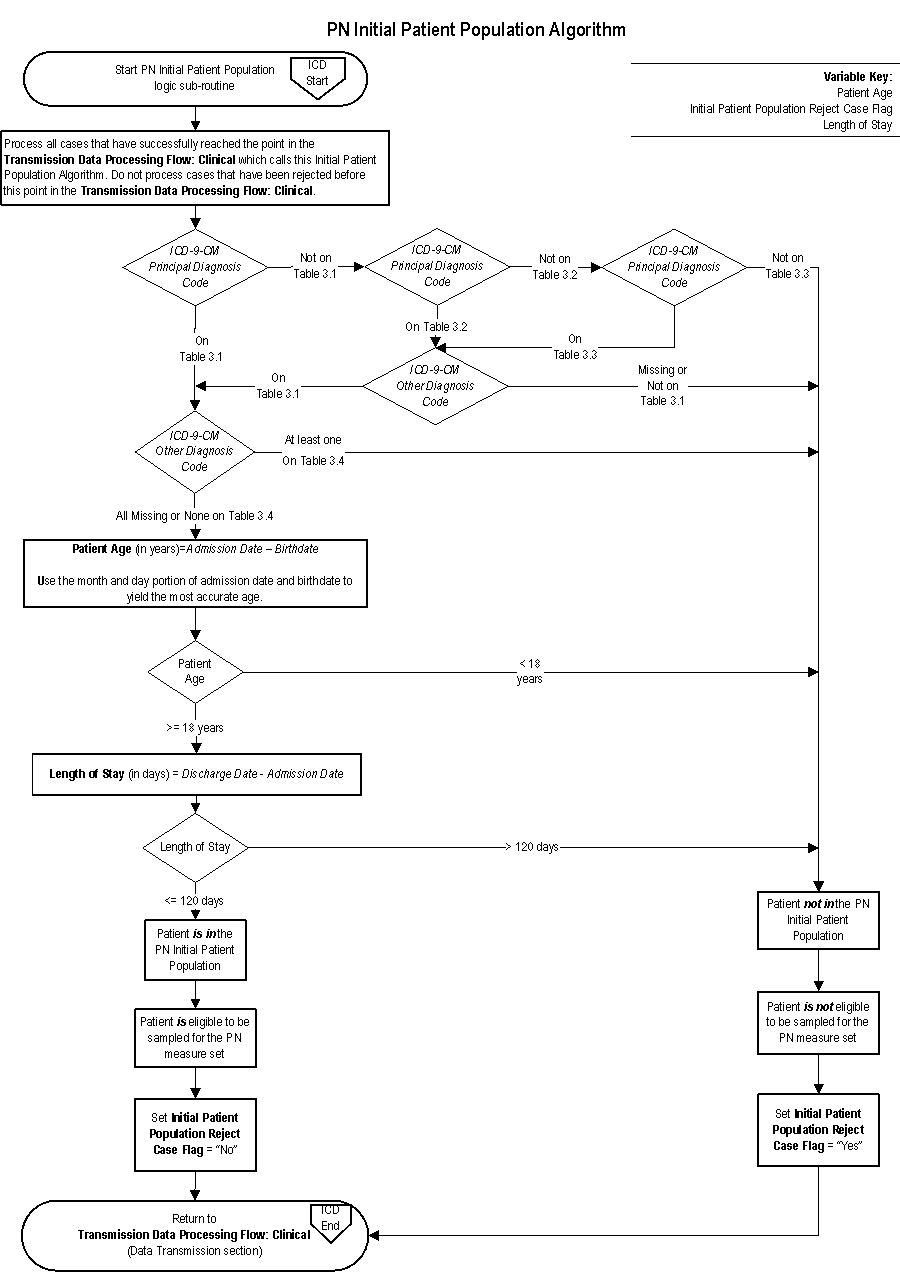
Pneumonia (PN) Initial Patient Population
The population of the PN measure set is identified using 5 data elements:
- ICD-9-CM Principal Diagnosis Code
- ICD-9-CM Other Diagnosis Codes
- Admission Date
- Birthdate
- Discharge Date
Patients admitted to the hospital for inpatient acute care are included in the PN Initial Patient Population and are eligible to be sampled if they have:
- An ICD-9-CM Principal Diagnosis Code for PN as defined in Appendix A, Table 3.1, NO ICD-9-CM Other Diagnosis Code of Cystic Fibrosis as defined in Appendix A, Table 3.4, a Patient Age (Admission Date minus Birthdate) greater than or equal to 18 years, and a Length of Stay (Discharge Date minus Admission Date) less than or equal to 120 days
OR
- An ICD-9-CM Principal Diagnosis Code of Septicemia or Respiratory Failure as defined in Appendix A, Table 3.2 and Table 3.3 accompanied by an ICD-9-CM Other Diagnosis Code of PN as defined in Appendix A, Table 3.1, NO ICD-9-CM Other Diagnosis Code of Cystic Fibrosis as defined in Appendix A, Table 3.4, a Patient Age (Admission Date minus Birthdate) greater than or equal to 18 years, and a Length of Stay (Discharge Date minus Admission Date) less than or equal to 120 days.
Note: The Joint Commission requires all PN measures to be collected and submitted as an entire measure set. Measures are specified in the aligned
Specifications Manual for National Hospital Inpatient Quality Measures as well as in the
Specifications Manual for Joint Commission National Quality Core Measures. The initial population and sampling should be determined for all of a hospital’s cases for the entire set, not at the individual measure level, even though individual measures are defined within the different manuals.
PN Sample Size Requirements
Hospitals that choose to sample have the option of sampling quarterly or sampling monthly. A hospital may choose to use a larger sample size than is required. Hospitals whose Initial Patient Population size is less than the minimum number of cases per quarter for the measure set cannot sample. Hospitals that have five or fewer PN discharges (both Medicare and non-Medicare combined) in a quarter are not required to submit PN patient level data to the QIO Clinical Warehouse and Joint Commissions Data Warehouse.
Regardless of the option used, hospital samples must be monitored to ensure that sampling procedures consistently produce statistically valid and useful data. Due to exclusions, hospitals selecting sample cases MUST submit AT LEAST the minimum required sample size.
The following sample size tables for each option automatically build in the number of cases needed to obtain the required sample sizes. For information concerning how to perform sampling, refer to the Population and Sampling Specifications section in this manual.
Quarterly Sampling
Hospitals performing quarterly sampling for PN must ensure that its Initial Patient Population and sample size meet the following conditions:
Quarterly Sample Size
Based on Initial Patient Population for the PN Measure Set
Monthly Sampling
Hospitals performing monthly sampling for PN must ensure that its Initial Patient Population and sample size meet the following conditions:
Monthly Sample Size
Based on Initial Patient Population for the PN Measure Set
Sample Size Examples
- Quarterly sampling:
- A hospital's Initial Patient Population size for the PN measure set is 171 PN discharges during the first quarter. The required quarterly sample is 60 cases.
- A hospital's PN Initial Patient Population size is 1,199 patients during the third quarter. The calculated sample size is 20% of the patient population or 240 cases for the quarter (twenty percent of 1,199 equals 239.8 rounded to the next highest whole number equals 240).
- A hospital's PN Initial Patient Population size is 3 patients during the second quarter. Submission of patient level data is not required. If the hospital chooses to submit patient level data:
- CMS: the quarterly sample size would be 1 - 3 cases for the quarter
- The Joint Commission: the required quarterly sample size would be 100% of the patient population or 3 cases for the quarter.
- Monthly sampling:
- A hospital's Initial Patient Population size for the PN measure set is 800 PN discharges during January. Twenty percent of 800 equals 160 pneumonia patients -- which exceeds the maximum monthly sample size condition (i.e., 81); thus, the required sample size would be at least 81 pneumonia patients for that month.
- A hospital's PN Initial Patient Population size is 399 patients during July. The required sample size is 20% of the patient population or 80 cases for the month (twenty percent of 399 equals 79.8 rounded to the next highest whole number equals 80).